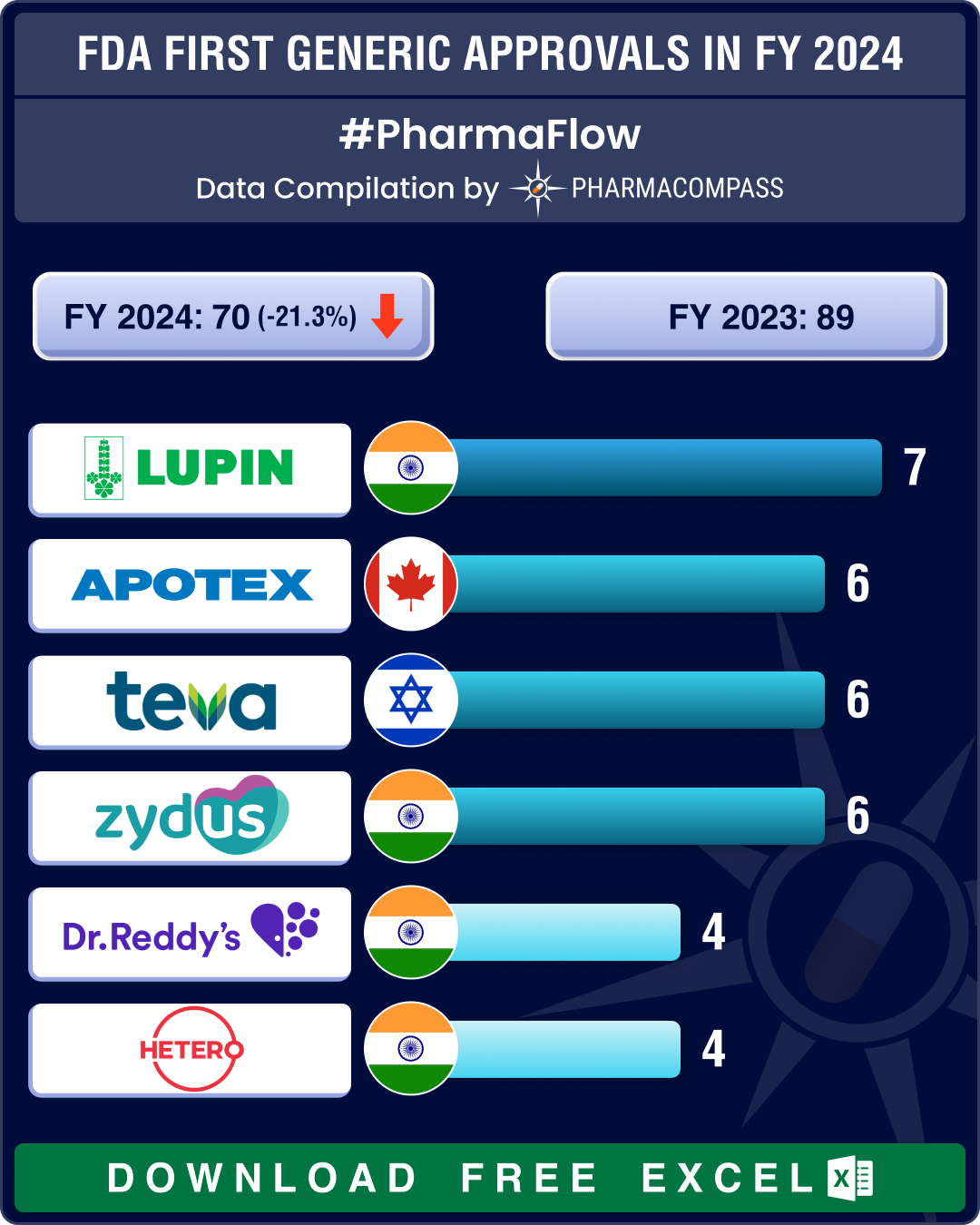
29 Apr 2025
// PRESS RELEASE
15 Apr 2025
// ECONOMICTIMES
14 Apr 2025
// ECONOMICTIMES
 KEY PRODUCTS
KEY PRODUCTS
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About
Product(s) under patent(s) are offered only for R&D purposes U/S 107A of the Patent Act and not for commercial sale
CPhI North America CPhI North America
Industry Trade Show
Attending
20-22 May, 2025
Industry Trade Show
Attending
26-28 August, 2025
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth #9.1B48
28-30 October, 2025
CONTACT DETAILS




Events
Webinars & Exhibitions
CPhI North America CPhI North America
Industry Trade Show
Attending
20-22 May, 2025
Industry Trade Show
Attending
26-28 August, 2025
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth #9.1B48
28-30 October, 2025
VLOG #PharmaReel
CORPORATE CONTENT #SupplierSpotlight
https://www.pharmacompass.com/radio-compass-blog/fda-s-first-generic-approvals-slump-21-in-2024-novartis-top-seller-entresto-cancer-blockbuster-tasigna-lead-2024-patent-cliff
https://www.pharmacompass.com/radio-compass-blog/fda-okays-50-new-drugs-in-2024-bms-cobenfy-lilly-s-kisunla-lead-pack-of-breakthrough-therapies
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-bora-polpharma-make-acquisitions-evonik-euroapi-porton-announce-technological-expansions
https://www.pharmacompass.com/radio-compass-blog/chinese-fda-registered-generic-facilities-gain-steam-india-maintains-lead-with-396-facilities
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-novo-s-parent-buys-catalent-for-us-16-5-bn-fujifilm-merck-kgaa-axplora-lonza-expand-capabilities
https://www.pharmacompass.com/radio-compass-blog/fda-approves-record-eight-biosimilars-in-h1-2024-okays-first-interchangeable-biosimilars-for-eylea
https://www.pharmacompass.com/radio-compass-blog/dmf-submissions-from-china-jump-42-as-india-continues-to-top-list-in-q1-2024

29 Apr 2025
// PRESS RELEASE
https://www.drreddys.com/cms/cms/sites/default/files/2025-04/DRL-Sanofi-%20Beyfortus%20press%20release-%20final%2028.04.2025%5B68%5D.pdf

15 Apr 2025
// ECONOMICTIMES
https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/sweet-surrender-sanofi-puts-star-insulin-brand-lantus-up-for-sale-eyes-rs-2000-crore-from-indian-pharma-giants/articleshow/120289524.cms

14 Apr 2025
// ECONOMICTIMES
https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/dr-reddys-slashing-jobs-to-cut-costs-by-25-asks-several-rs-1-crore-earners-to-resign/articleshow/120277193.cms

12 Apr 2025
// ECONOMICTIMES
https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/weight-is-over-pharma-companies-race-to-make-weight-loss-drug-semaglutide/articleshow/120215666.cms

09 Apr 2025
// REUTERS
https://www.reuters.com/business/healthcare-pharmaceuticals/indian-pharma-stocks-decline-after-trump-again-threatens-tariffs-2025-04-09/https://www.reuters.com/business/healthcare-pharmaceuticals/indian-pharma-stocks-decline-after-trump-again-threatens-tariffs-2025-04-09/

08 Apr 2025
// INDPHARMAPOST
https://www.indianpharmapost.com/news/dr-reddys-laboratories-gets-rs-2395-crore-show-cause-notice-from-it-authority-16983
Regulatory Info : RX
Registration Country : USA
Dosage Form : TABLET;ORAL
Brand Name : ABIRATERONE ACETATE
Dosage Strength : 500MG
Packaging :
Approval Date : 2023-09-01
Application Number : 208416
Regulatory Info : RX
Registration Country : USA
Regulatory Info : Lead Market Dossiers- Filed
Registration Country : India
Dosage Form : Oral Solid Dosage Form
Brand Name :
Dosage Strength : 500MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Lead Market Dossiers- Filed
Registration Country : India
Regulatory Info : RX
Registration Country : USA
Dosage Form : TABLET;ORAL
Brand Name : ABIRATERONE ACETATE
Dosage Strength : 250MG
Packaging :
Approval Date : 2020-05-18
Application Number : 208416
Regulatory Info : RX
Registration Country : USA
Regulatory Info : Lead Market Dossiers- Approved in US
Registration Country : India
Dosage Form : Tablet
Brand Name :
Dosage Strength : 250MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Lead Market Dossiers- Approved in US
Registration Country : India
Regulatory Info : Lead Market Dossiers- Filed
Registration Country : India
Dosage Form : Oral Solid Dosage Form
Brand Name :
Dosage Strength : 250MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Lead Market Dossiers- Filed
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Dosage Form : CAPSULE;ORAL
Brand Name : SECTRAL
Dosage Strength : EQ 400MG BASE **Federa...
Packaging :
Approval Date : 1984-12-28
Application Number : 18917
Regulatory Info : DISCN
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Dosage Form : CAPSULE;ORAL
Brand Name : SECTRAL
Dosage Strength : EQ 200MG BASE **Federa...
Packaging :
Approval Date : 1984-12-28
Application Number : 18917
Regulatory Info : DISCN
Registration Country : USA
Regulatory Info : RX
Registration Country : USA
Dosage Form : CAPSULE;ORAL
Brand Name : BUTALBITAL AND ACETAMINOP...
Dosage Strength : 300MG;50MG
Packaging :
Approval Date : 2017-12-27
Application Number : 207313
Regulatory Info : RX
Registration Country : USA
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; BUTALBITAL; CAFFEINE
Dosage Form : CAPSULE;ORAL
Brand Name : BUTALBITAL, ACETAMINOPHEN...
Dosage Strength : 300MG;50MG;40MG
Packaging :
Approval Date : 2019-12-17
Application Number : 210817
Regulatory Info : RX
Registration Country : USA
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; BUTALBITAL; CAFFEINE
Dosage Form : CAPSULE;ORAL
Brand Name : BUTALBITAL, ACETAMINOPHEN...
Dosage Strength : 325MG;50MG;40MG
Packaging :
Approval Date : 1986-03-17
Application Number : 89007
Regulatory Info : RX
Registration Country : USA
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients
Inspections and registrations
ABOUT THIS PAGE
Dr. Reddy's Laboratories is a supplier offers 206 products (APIs, Excipients or Intermediates).
Find a price of Atorvastatin bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Capecitabine bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Cetirizine Dihydrochloride bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Clopidogrel bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Fexofenadine Hydrochloride bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Finasteride bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Gemcitabine bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Levetiracetam bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Montelukast Sodium bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Nizatidine bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Ondansetron Hydrochloride bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Quetiapine Hemifumarate bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Rabeprazole Sodium bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Risperidone bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Rivaroxaban bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Valsartan bulk with DMF, CEP, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Amlodipine Besylate bulk with DMF, CEP, JDMF offered by Dr. Reddy's Laboratories
Find a price of Atorvastatin bulk with DMF, CEP, JDMF offered by Dr. Reddy's Laboratories
Find a price of Azacitidine bulk with DMF, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Dasatinib bulk with DMF, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Esomeprazole Magnesium bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Famotidine bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Glimepiride bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Ketorolac Trometamol bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Lenalidomide bulk with DMF, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Losartan Potassium bulk with DMF, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Naproxen bulk with DMF, CEP, JDMF offered by Dr. Reddy's Laboratories
Find a price of Naratriptan Hydrochloride bulk with DMF, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Omeprazole bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Omeprazole Magnesium bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Pantoprazole Sodium bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Pioglitazone Hydrochloride bulk with DMF, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Rabeprazole Sodium bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Raloxifene Hydrochloride bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Rivastigmine Tartrate bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Sitagliptin Phosphate bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Sugammadex Sodium bulk with DMF, JDMF, WC offered by Dr. Reddy's Laboratories
Find a price of Ticagrelor bulk with DMF, CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Abiraterone Acetate bulk with DMF, JDMF offered by Dr. Reddy's Laboratories
Find a price of Apalutamide bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Apixaban bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Apremilast bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Atomoxetin Hydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Atorvastatin bulk with DMF, JDMF offered by Dr. Reddy's Laboratories
Find a price of Bendamustine Hydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Bortezomib bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Cabazitaxel bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Carfilzomib bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Cinacalcet Hydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Dabigatran Etexilate Mesylate bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Dapagliflozin bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Dapagliflozin Propanediol Monohydrate bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Decitabine bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Desloratadine bulk with CEP, WC offered by Dr. Reddy's Laboratories
Find a price of Dutasteride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Edaravone bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Elagolix Sodium bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Enzalutamide bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Eslicarbazepine Acetate bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Eszopiclone bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Fondaparinux Sodium bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Gatifloxacin bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Iron Sucrose bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Lenvatinib Mesylate bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Levocetirizine Dihydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Levofloxacin Hemihydrate bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Linagliptin bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Lomustine bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Losartan Potassium bulk with DMF, JDMF offered by Dr. Reddy's Laboratories
Find a price of Lurasidone Hydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Metoprolol Succinate bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Mirabegron bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Moxifloxacin Hydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Naproxen bulk with DMF, CEP offered by Dr. Reddy's Laboratories
Find a price of Naproxen Sodium bulk with DMF, CEP offered by Dr. Reddy's Laboratories
Find a price of Nilotinib bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Omeprazole Magnesium bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Ondansetron bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Palonosetron bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Pantoprazole Sodium bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Pemetrexed Disodium bulk with DMF, CEP offered by Dr. Reddy's Laboratories
Find a price of Permethrin bulk with DMF, CEP offered by Dr. Reddy's Laboratories
Find a price of Pomalidomide bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Posaconazole bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Pregabalin bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Ramipril bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Ranolazine bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Roxadustat bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Sacubitril-Valsartan bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Sitagliptin Hydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Sumatriptan bulk with DMF, CEP offered by Dr. Reddy's Laboratories
Find a price of Terbinafine Hydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Tizanidine HCl bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Ziprasidone Hydrochloride bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Zoledronic Acid bulk with DMF, WC offered by Dr. Reddy's Laboratories
Find a price of Apalutamide bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Apremilast bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Atomoxetin Hydrochloride bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Atorvastatin bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Bempedoic Acid bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Benztropine bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Cabozantinib bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Capecitabine bulk with CEP offered by Dr. Reddy's Laboratories
Find a price of Cetirizine Dihydrochloride bulk with CEP offered by Dr. Reddy's Laboratories
Find a price of Dabigatran Etexilate Mesylate bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Dasatinib bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Decitabine bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Donepezil bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Doxazosin Mesylate bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Empagliflozin bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Eribulin bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Esomeprazole Magnesium bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Ezetimibe bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Ferric Carboxymaltose bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Fexofenadine Hydrochloride bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Fosaprepitant bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Glatiramer Acetate bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Granisetron bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Ketorolac Trometamol bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Lacidipine bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Lenalidomide bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Lifitegrast bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Lubiprostone bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Lumateperone Tosylate bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Memantine Hydrochloride bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Metoprolol Succinate bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Midostaurin bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Mirabegron bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Naproxen bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Nilotinib bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Olaparib bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Omeprazole bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Ondansetron Hydrochloride bulk with JDMF offered by Dr. Reddy's Laboratories
Find a price of Oxaprozin bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Palbociclib bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Pazopanib Hydrochloride bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Pemetrexed Ditromethamine bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Pregabalin bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Rabeprazole Sodium bulk with CEP offered by Dr. Reddy's Laboratories
Find a price of Relugolix bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Ripretinib bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Roxadustat bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Sitagliptin Hydrochloride bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Sitagliptin Phosphate bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Sumatriptan bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Tafamidis Meglumine bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Testosterone bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Tofacitinib Citrate bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Travoprost bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Valsartan bulk with JDMF offered by Dr. Reddy's Laboratories
Find a price of Varenicline Tartrate bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Venetoclax bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Vonoprazan Fumarate bulk with DMF offered by Dr. Reddy's Laboratories
Find a price of Zoledronic Acid bulk with WC offered by Dr. Reddy's Laboratories
Find a price of Acalabrutinib bulk offered by Dr. Reddy's Laboratories
Find a price of Adagrasib bulk offered by Dr. Reddy's Laboratories
Find a price of Amlodipine Besylate bulk offered by Dr. Reddy's Laboratories
Find a price of Apixaban bulk offered by Dr. Reddy's Laboratories
Find a price of Atorvastatin bulk offered by Dr. Reddy's Laboratories
Find a price of Cetirizine Dihydrochloride bulk offered by Dr. Reddy's Laboratories
Find a price of Ciprofloxacin Hydrochloride bulk offered by Dr. Reddy's Laboratories
Find a price of Clopidogrel bulk offered by Dr. Reddy's Laboratories
Find a price of Dapagliflozin Propanediol Monohydrate bulk offered by Dr. Reddy's Laboratories
Find a price of Darolutamide bulk offered by Dr. Reddy's Laboratories
Find a price of Deucravacitinib bulk offered by Dr. Reddy's Laboratories
Find a price of Dimethyl Fumarate bulk offered by Dr. Reddy's Laboratories
Find a price of Dutasteride bulk offered by Dr. Reddy's Laboratories
Find a price of Edoxaban Tosylate bulk offered by Dr. Reddy's Laboratories
Find a price of Elinzanetant bulk offered by Dr. Reddy's Laboratories
Find a price of Empagliflozin bulk offered by Dr. Reddy's Laboratories
Find a price of Fezolinetant bulk offered by Dr. Reddy's Laboratories
Find a price of Fruquintinib bulk offered by Dr. Reddy's Laboratories
Find a price of Gemcitabine bulk offered by Dr. Reddy's Laboratories
Find a price of Ketorolac Trometamol bulk offered by Dr. Reddy's Laboratories
Find a price of Liraglutide bulk offered by Dr. Reddy's Laboratories
Find a price of Mavacamten bulk offered by Dr. Reddy's Laboratories
Find a price of Moxifloxacin Hydrochloride bulk offered by Dr. Reddy's Laboratories
Find a price of Naproxen bulk offered by Dr. Reddy's Laboratories
Find a price of Naproxen Sodium bulk offered by Dr. Reddy's Laboratories
Find a price of Niraparib Tosylate bulk offered by Dr. Reddy's Laboratories
Find a price of Nizatidine bulk offered by Dr. Reddy's Laboratories
Find a price of Omeprazole bulk offered by Dr. Reddy's Laboratories
Find a price of Perfluorohexyloctane bulk offered by Dr. Reddy's Laboratories
Find a price of Pirtobrutinib bulk offered by Dr. Reddy's Laboratories
Find a price of Pregabalin bulk offered by Dr. Reddy's Laboratories
Find a price of Resmetirom bulk offered by Dr. Reddy's Laboratories
Find a price of Rimegepant Sulfate bulk offered by Dr. Reddy's Laboratories
Find a price of Risperidone bulk offered by Dr. Reddy's Laboratories
Find a price of Ritlecitinib bulk offered by Dr. Reddy's Laboratories
Find a price of Ropinirole bulk offered by Dr. Reddy's Laboratories
Find a price of Ruxolitinib Phosphate bulk offered by Dr. Reddy's Laboratories
Find a price of Sacubitril-Valsartan bulk offered by Dr. Reddy's Laboratories
Find a price of Salcaprozate Sodium bulk offered by Dr. Reddy's Laboratories
Find a price of Siponimod bulk offered by Dr. Reddy's Laboratories
Find a price of Siponimod fumarate bulk offered by Dr. Reddy's Laboratories
Find a price of Sugammadex Sodium bulk offered by Dr. Reddy's Laboratories
Find a price of Tafamidis bulk offered by Dr. Reddy's Laboratories
Find a price of Terbinafine Hydrochloride bulk offered by Dr. Reddy's Laboratories
Find a price of Ticagrelor bulk offered by Dr. Reddy's Laboratories
Find a price of Treprostinil Sodium bulk offered by Dr. Reddy's Laboratories
Find a price of Tucatinib bulk offered by Dr. Reddy's Laboratories
Find a price of Valsartan bulk offered by Dr. Reddy's Laboratories
Find a price of Voclosporin bulk offered by Dr. Reddy's Laboratories
Find a price of Xanomeline bulk offered by Dr. Reddy's Laboratories
Find a price of Zafirlukast bulk offered by Dr. Reddy's Laboratories
Find a price of Zanubrutinib bulk offered by Dr. Reddy's Laboratories