Company Bio #AboutSupplier
View Mission CDMO's services for oral and topical drug delivery & browse its CDMO capabilities for tablets, liquids, creams & gels on PharmaCompass.
Impressions : 706
View Sharp's clinical trial supply & contract pharmaceutical packaging services for injectables and oral dosage forms on PharmaCompass.
Impressions : 2579
View Temad's portfolio of Narcotic & Non-Narcotic APIs, Intermediates & Finished Products & explore Temad's manufacturing activities on PharmaCompass.
Impressions : 2650
View Kewpie's pharmaceutical grade Sodium hyaluronate & browse its different grades of Hyaluronic Acid for injectables & eye drops on PharmaCompass.
Impressions : 4526
View Sharp's clinical trial supply & contract pharmaceutical packaging services, including blisters, bottles, sachets, pouches & PFS on PharmaCompass.
Impressions : 3727
View Metrochem API's portfolio of APIs, Intermediates & Pellets (Semi-finished formulations) & explore Metrochem's R&D activities on PharmaCompass.
Impressions : 3329
View Proveris' drug development & contract analytical services for orally inhaled, dry powder & nasal drug products (OINDPs) on PharmaCompass.
Impressions : 1533
Overview of Mikart's CDMO services, such as controlled substance formulations, oral solids & non-sterile liquid dosage forms on PharmaCompass.
Impressions : 2008
Overview of PMC Isochem's polyamino acid (PAA) based polymers, polymer conjugates, polypeptides, polyamides for drug delivery on PharmaCompass.
Impressions : 2192
Overview of peptide development & contract manufacturing services & more on CBL's CDMO & CMO services for non-GMP & cGMP peptides on PharmaCompass.
Impressions : 5655
Overview of PMC Isochem's Vitamin E TPGS (Tocophersolan), a multirole excipient for pharmaceutical drug delivery, on PharmaCompass.
Impressions : 2563
Overview of high potency APIs or HPAPI contract manufacturing services & more on Aspen API's CDMO services for cytotoxic HPAPIs on PharmaCompass.
Impressions : 2731
Overview of small molecule drug development & contract manufacturing & more on EUROAPI's CDMO services for complex & small molecules on PharmaCompass.
Impressions : 3135
Overview of CDMO Services of EUROAPI, such as small molecule APIs, peptides, microbial fermentation, micronization & spray drying on PharmaCompass.
Impressions : 2615
View EUROAPI's portfolio of APIs like small molecules, peptides, steroids, opiates & controlled substances & browse its CDMO services on PharmaCompass
Impressions : 7740
Overview of highly potent or high potency API (HPAPI) & more on Gentec's contract manufacturing services for high potency drugs on PharmaCompass.
Impressions : 1636
Overview of custom peptide synthesis & contract manufacturing services & more on Aspen API's CDMO & CMO services for cGMP peptides on PharmaCompass.
Impressions : 4538
View Gentec's portfolio of various APIs including High potency APIs & Prostaglandins & browse its Contract manufacturing services on PharmaCompass.
Impressions : 2695
View Aspen API's portfolio of various APIs like peptide APIs, HPAPIs, narcotics & biochemical APIs & browse its CDMO services on PharmaCompass.
Impressions : 2957
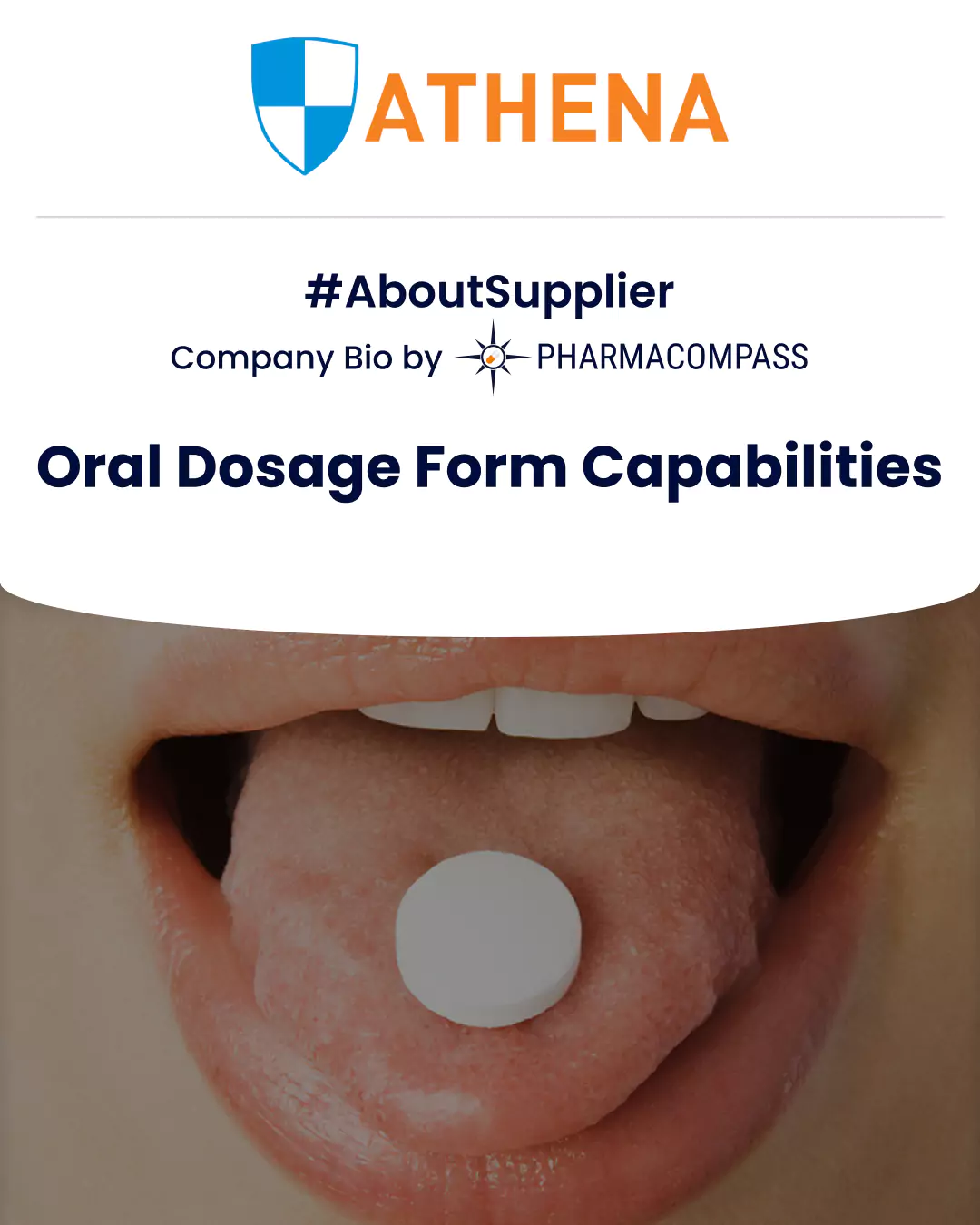
Overview of oral solid dosage forms (OSD) like orodispersible tablets (ODTs) & more on Athena's CDMO services for oral drug delivery on PharmaCompass.
Impressions : 1537
Overview of Athena's oral formulations including effervescent powder, granules & tablets, Oral Disintegrating Tablets (ODT) & more on PharmaCompass.
Impressions : 980
Overview of stick-packs including liquid & powder oral sticks, contract packaging & more on Athena, a CDMO for stick packs, on PharmaCompass.
Impressions : 1719
Looking for highly potent drug substances or HPAPIs? Find a CDMO, CMO offering high potent complex APIs, intermediates & cytotoxics on PharmaCompass.
Impressions : 1856
Looking for API custom synthesis services? Find Minakem, a CDMO offering custom APIs, intermediates, fine chemicals, HPAPIs, etc. on PharmaCompass.
Impressions : 927
View Minakem's portfolio of APIs & pharmaceutical intermediates & browse Minakem's CDMO services including custom API manufacturing on PharmaCompass.
Impressions : 980
View Pfizer CentreOne's portfolio of APIs & intermediate products & also view Pfizer CentreOne's CDMO / CMO services on PharmaCompass.
Impressions : 1584


 Market Place
Market Place Sourcing Support
Sourcing Support