Data Compilation #PharmaFlow
FDA’s December 2025 OPOE list features 784 prescription drugs, 73 OTC drugs
This
week, PharmaCompass brings you key highlights of the US Food and Drug Administration’s D
Top news of 2025: Drugmakers invest in US capacities, agree to lower Medicaid prices; Pfizer buys obesity-focused biotech Metsera
The year 2025 was an eventful one, marked by increased trade
tensions, tariff threats, accelerated
CDMO Activity Tracker: Lupin’s CDMO commissions oncology block in India; Cambrex, Axplora, Hovione expand operations
The global contract development and
manufacturing organization (CDMO) market has been growing at a
Antibiotic market to reach US$ 74.07 billion by 2033; FDA approves GSK’s Blujepa for uncomplicated UTIs
The global market
for antibiotics has been witnessing several challenges. First, there is growing
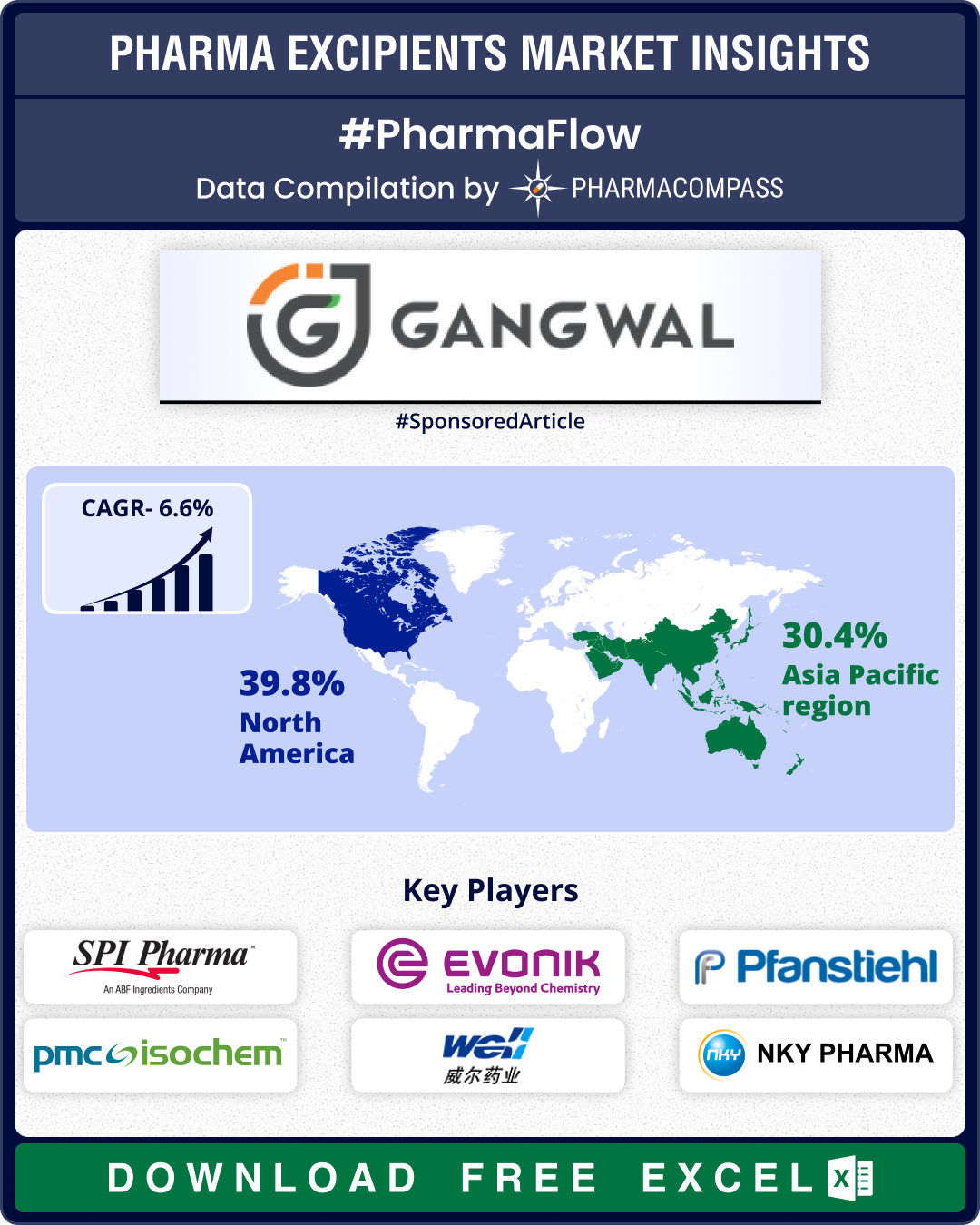
Excipient Market Overview: Evonik launches high-purity excipients; India mandates disclosures from March 2026
The global pharmaceutical excipients market continued to evolve in the third quarter (Q3) of 2025, s
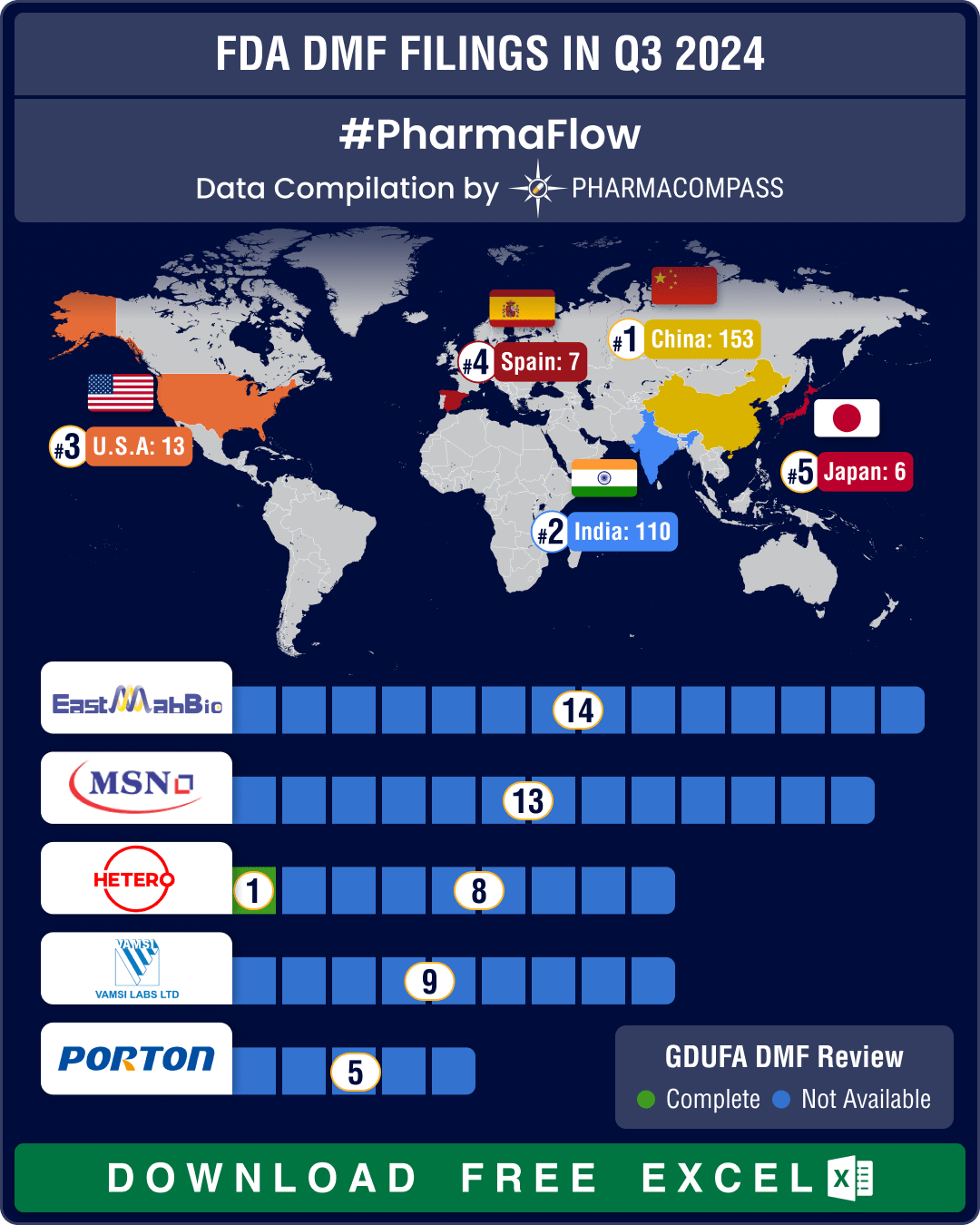
DMF filings rise 4.5% in Q3 2025; China holds lead, India records 20% growth in submissions
The
third quarter (Q3) of 2025 witnessed a steady rise in Drug Master File (DMF) submissions to the
CDMO Activity Tracker: Veranova, ChemExpress invest in ADC facilities; Cohance to set up oligonucleotide facility in India
The contract development and manufacturing organization (CDMO) sector has emerged as a key partner i
Global API micronization market set to surge 49% by 2030, led by specialized industry pioneers
Precise particle size control has become the cornerstone of modern pharmaceutical manufacturing. Wit
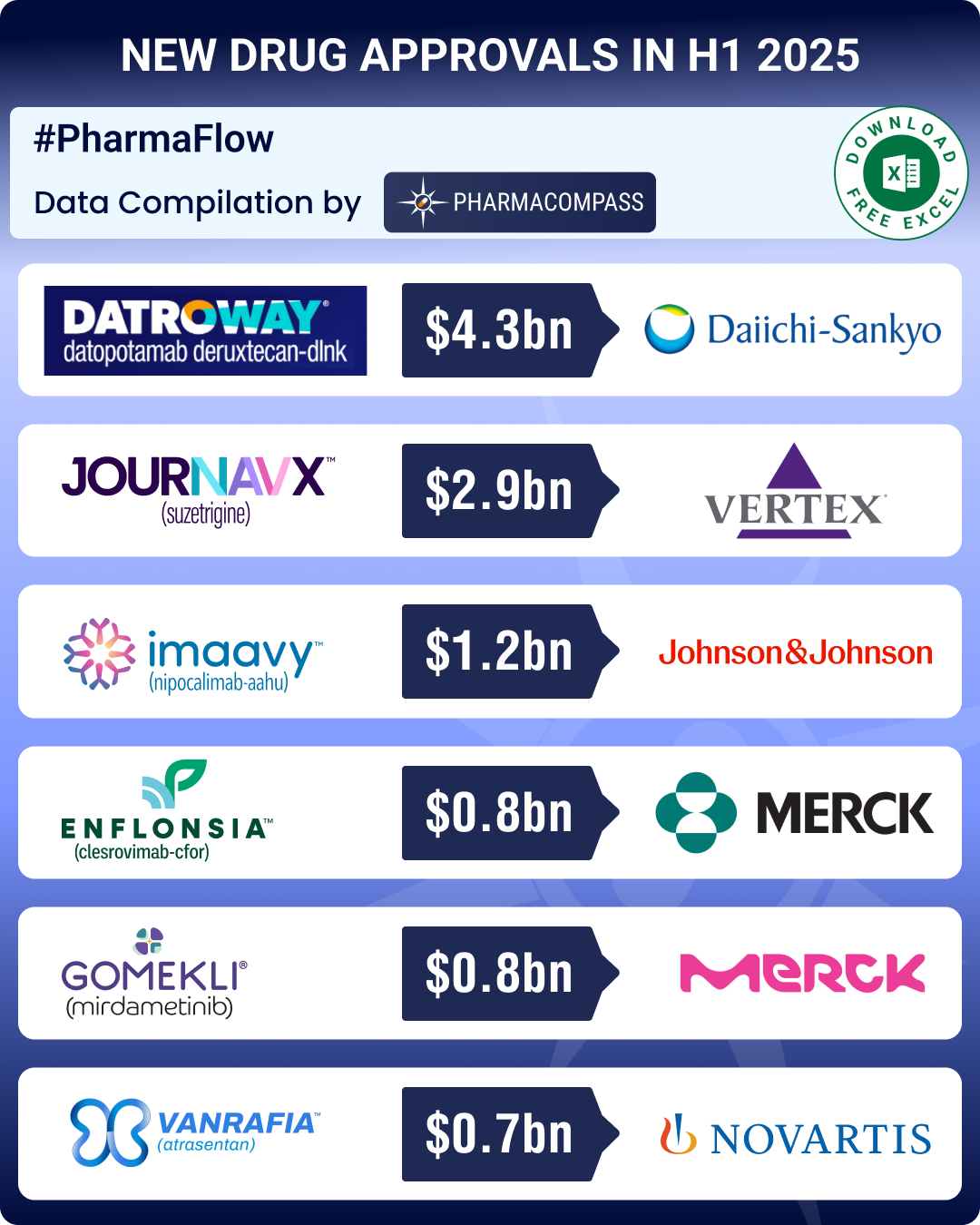
FDA approvals drop 24% in H1 2025; GSK’s UTI med, Vertex’s non-opioid painkiller lead pack of first-in-class meds
It has been a turbulent year for the US
Food and Drug Administration (FDA), marked by reduction
CDMO Activity Tracker: Veranova, Carbogen lead ADC investments; Axplora, Polfa Tarchomin, Famar expand European footprint
During the second quarter (Q2) of 2025, contract development and manufacturing organizations (CDMOs)
J&J’s Intra‑Cellular buyout, BMS’ oncology gambit, Sanofi’s Blueprint acquisition drive mega deals in H1 2025
The pharmaceutical industry has witnessed a wave of mergers, acquisitions, and strategic partnership
Excipient Market Overview: Roquette announces restructuring post IFF Pharma buyout; WHO, FDA advance regulatory frameworks
The pharmaceutical excipients market saw significant strategic
consolidations, technological develo
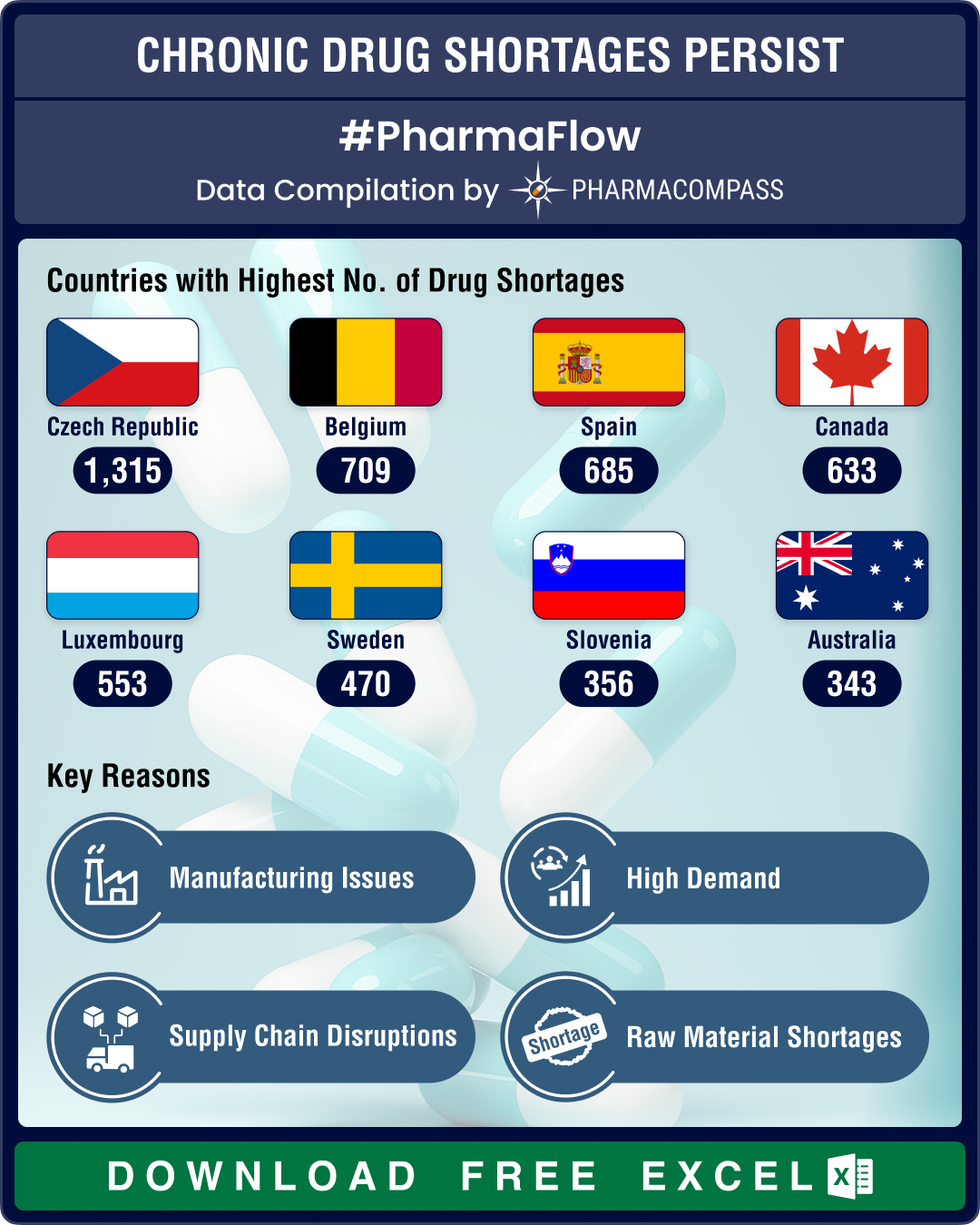
US drug shortages reduce 16% YoY in Q1 2025; CNS drugs, antimicrobials face highest scarcities
The pharmaceutical industry in the United States continued to
grapple with drug shortages during th
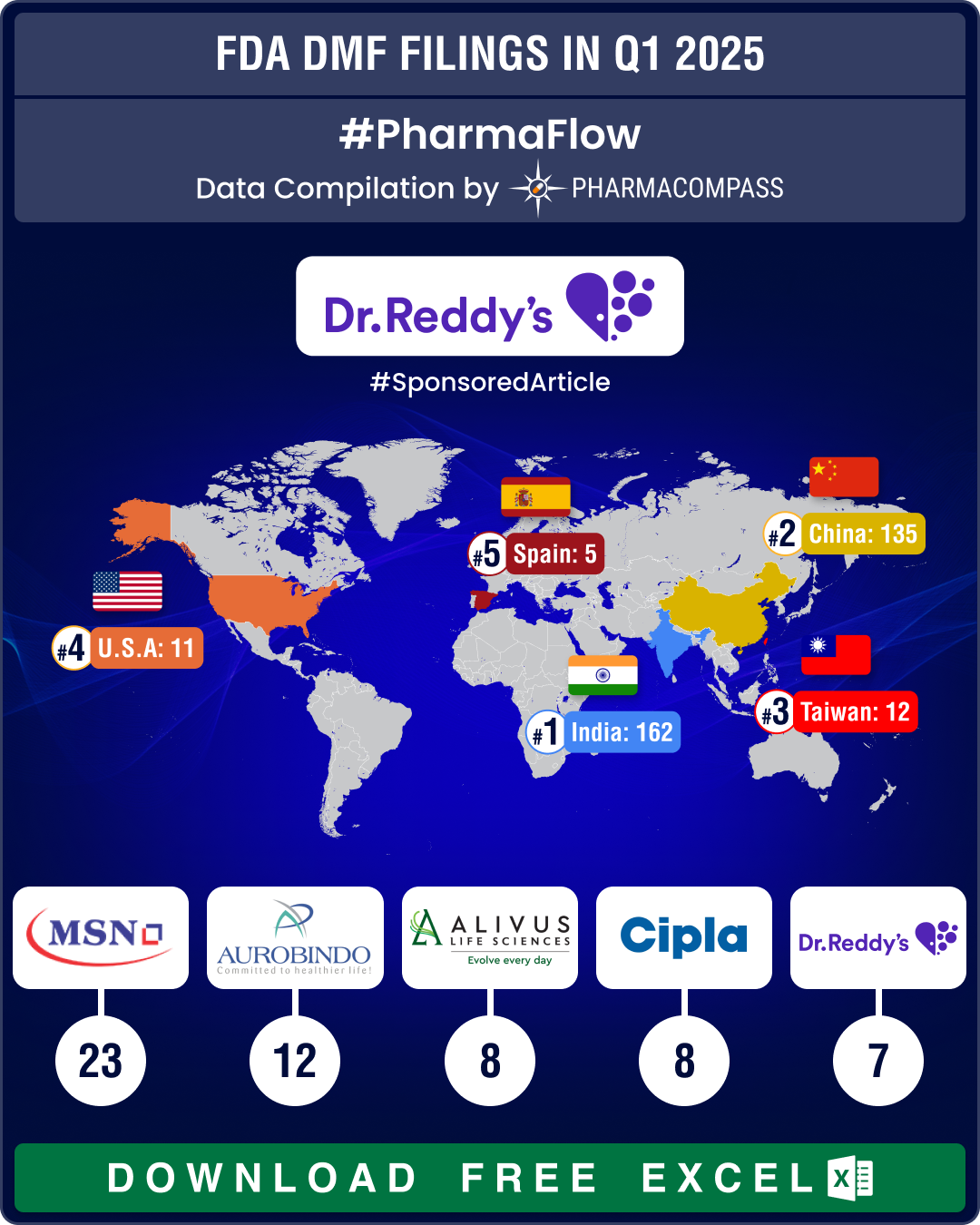
DMF filings surge 44% in Q1 2025; India tops list with 51% rise in year-on-year submissions
The first quarter (Q1) of 2025 witnessed an impressive surge in Drug Master File (DMF) submissions t
Top Pharma Companies & Drugs in 2024: Merck’s Keytruda maintains top spot as Novo’s semaglutide nips at its heels
In 2024, Big Pharma players consolidated
and maintained their dominance, even as innovation continu
How Trump’s tariffs on imported drugs can hurt supply chains, consumers and industry
Ever since Donald Trump has moved into the White House for a second term, there has been turmoil acr
CDMO Activity Tracker: Axplora enhances ADC capacities, Quotient beefs up HPAPI capabilities; Evonik, EUROAPI forge deals
The contract development and
manufacturing organization (CDMO) sector witnessed significant activit
Molecular glue degraders: Lilly, AbbVie sign billion-dollar deals; BMS leads with three late-stage drugs
This week, we delve into molecular glue degraders (MGDs), one of the most promising frontiers in dru
AI drug discovery market to grow 30% CAGR, to reach US$ 35 bn by 2034; Novo, Lilly, BMS forge deals
Artificial intelligence (AI) is emerging
as a transformative force in drug discovery and developmen
Top first-in-class drug candidates of 2025: Ionis’ donidalorsen, Sanofi’s fitusiran, Cytokinetics’ aficamten await FDA approval
First‑in‑class drugs are therapies with entirely new approaches that improve patient out
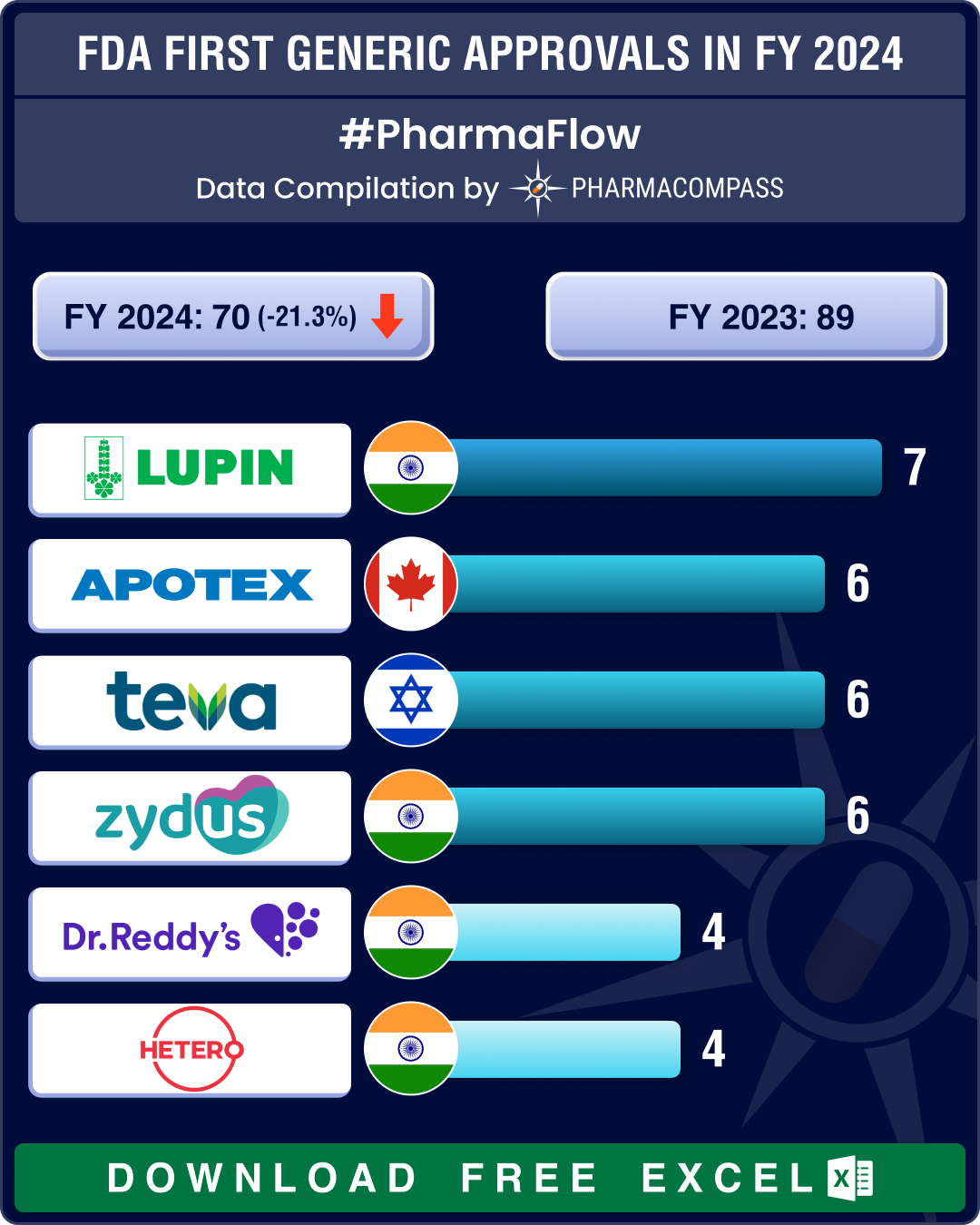
FDA’s first generic approvals slump 21% in 2024; Novartis’ top seller Entresto, cancer blockbuster Tasigna lead 2024 patent cliff
A watershed moment in the journey of a drug is when it transitions from being a patented, high‐
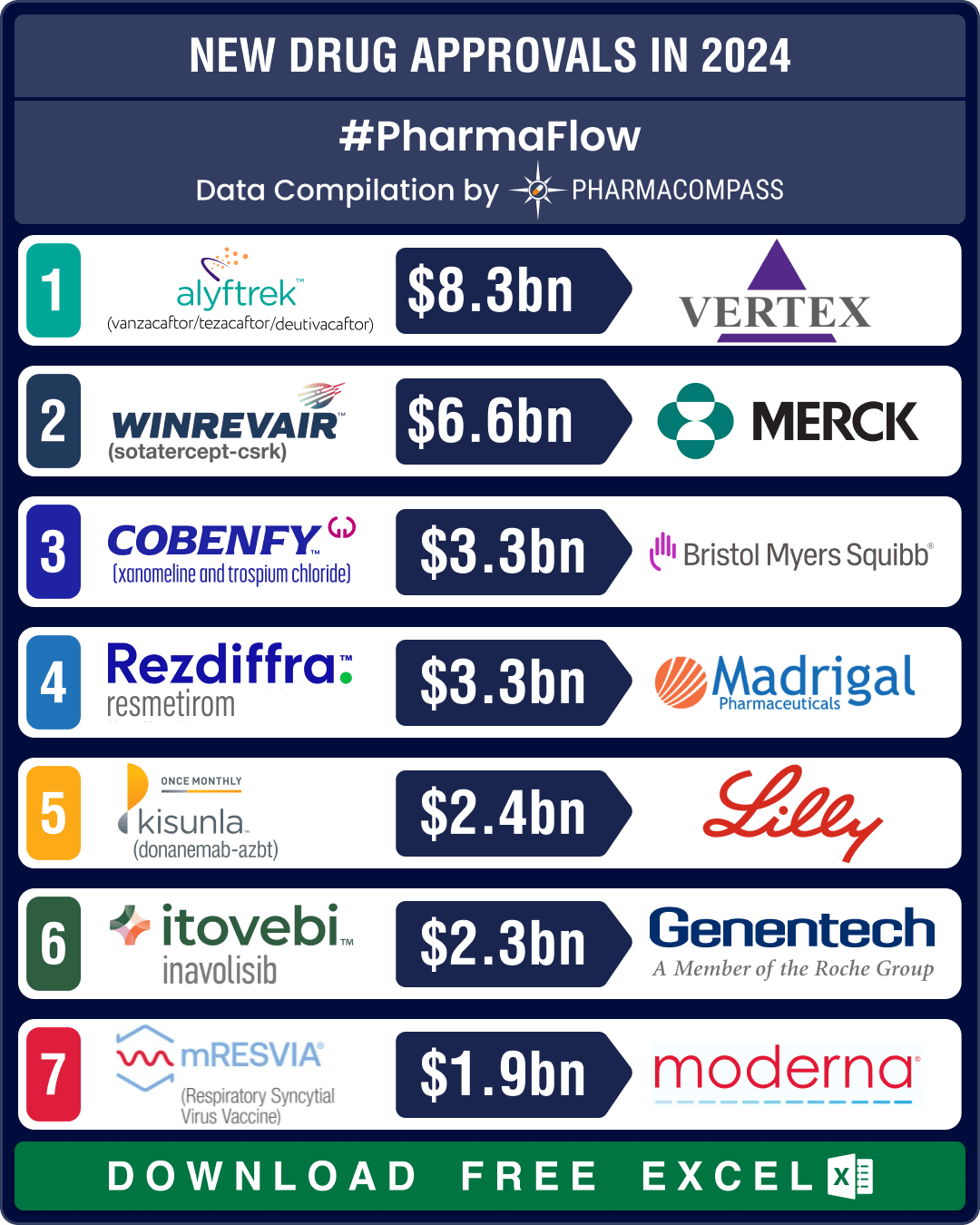
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though
BMS, J&J, Bayer lead 25,000+ pharma layoffs in 2024; Amylyx, FibroGen, Kronos Bio hit by trial failures, cash crunch
Since 2022, there has been a significant surge in layoffs by pharmaceutical and biotech companies. W
FDA’s landmark approvals of BMS’ schizo med, Madrigal’s MASH drug, US$ 16.5 bn Catalent buyout make it to top 10 news of 2024
The year 2024 was marked by some landmark drug approvals in the areas of schizophrenia, metabolic dy
CDMO Activity Tracker: Bora, PolPharma make acquisitions; Evonik, EUROAPI, Porton announce technological expansions
The contract development and
manufacturing organization (CDMO) space continued to grow at an impres
Chinese FDA-registered generic facilities gain steam, India maintains lead with 396 facilities
Every year, the US Food and Drug Administration (FDA) publishes the user fee amounts it will collect
Medical Breakthroughs in 2024: Alzheimer’s, schizophrenia, COPD, MASH see pathbreaking treatments
This year has seen pivotal advancements in medical innovation. The US Food and Drug Administration (


 Market Place
Market Place Sourcing Support
Sourcing Support