19 Dec 2025
// PRESS RELEASE
18 Dec 2025
// PRESS RELEASE
18 Dec 2025
// PRESS RELEASE
 KEY PRODUCTS
KEY PRODUCTS KEY SERVICES
KEY SERVICES
Aspen API. More than just an API™
About
Industry Trade Show
Attending
23-26 March, 2026
Industry Trade Show
Attending
21-23 April, 2026
Industry Trade Show
Booth #814 Level 2
11-14 May, 2026
CONTACT DETAILS





Events
Webinars & Exhibitions
Industry Trade Show
Attending
23-26 March, 2026
Industry Trade Show
Attending
21-23 April, 2026
Industry Trade Show
Booth #814 Level 2
11-14 May, 2026
CORPORATE CONTENT #SupplierSpotlight
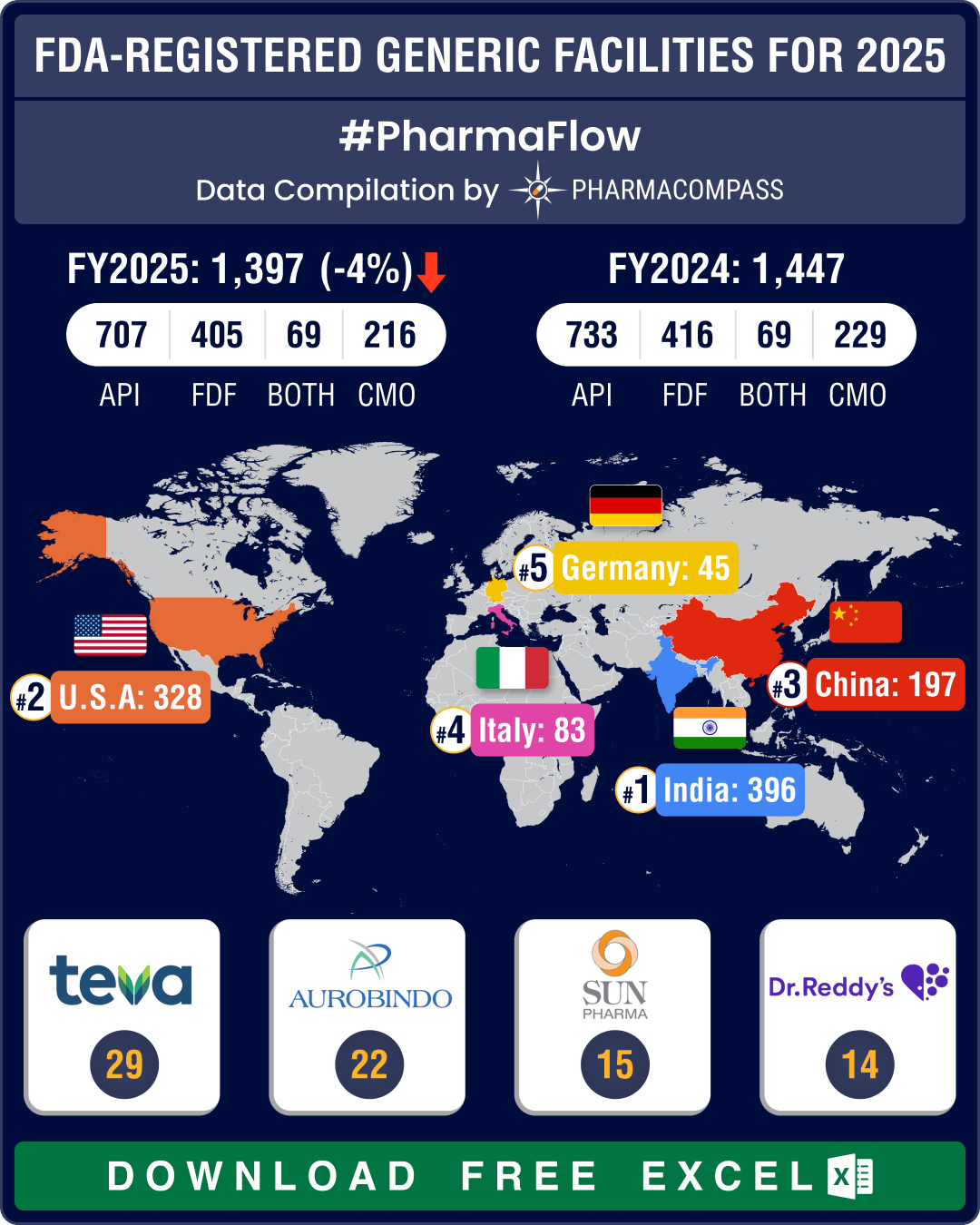
https://www.pharmacompass.com/radio-compass-blog/chinese-fda-registered-generic-facilities-gain-steam-india-maintains-lead-with-396-facilities
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-novo-s-parent-buys-catalent-for-us-16-5-bn-fujifilm-merck-kgaa-axplora-lonza-expand-capabilities

19 Dec 2025
// PRESS RELEASE
https://www.aspenapi.com/news/aspen-api-pioneering-sustainable-peptide-manufacturing/

18 Dec 2025
// PRESS RELEASE
https://www.aspenapi.com/news/strong-reinforcement-of-the-oss-pharma-cluster-through-the-arrival-of-aspen-oss-at-pivot-park/

18 Dec 2025
// PRESS RELEASE
https://www.aspenapi.com/news/clascoterone-and-segesterone-new-development-opportunities-in-our-aspen-api-steroid-portfolio/

30 Sep 2025
// PRESS RELEASE
https://www.aspenapi.com/news/yes-hashtagestradiol-is-added-to-our-list-of-approved-cadifas/

28 Feb 2025
// PRESS RELEASE

11 Feb 2025
// PRESS RELEASE
https://www.aspenapi.com/news/this-project-aimed-to-optimize-the-purification-process-of-desogestrel/
Certificate Numbers : R1-CEP 2017-171 - Rev 00
Status : Valid
Issue Date : 2023-02-13
Type : Chemical
Substance Number : 369
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Fine Chemicals Corporation PTY Ltd
Certificate Numbers : R1-CEP 2010-052 - Rev 01
Status : Valid
Issue Date : 2019-01-18
Type : Chemical
Substance Number : 74
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Fine Chemicals Corporation PTY Ltd
Certificate Numbers : CEP 2021-385 - Rev 01
Status : Valid
Issue Date : 2024-02-06
Type : Chemical
Substance Number : 74
Certificate Numbers : R1-CEP 2009-300 - Rev 00
Status : Valid
Issue Date : 2016-03-16
Type : Chemical
Substance Number : 1717
Certificate Numbers : R2-CEP 1995-001 - Rev 03
Status : Valid
Issue Date : 2014-01-30
Type : Chemical
Substance Number : 821
Certificate Numbers : CEP 2009-171 - Rev 02
Status : Valid
Issue Date : 2025-04-25
Type : Chemical
Substance Number : 1614
Certificate Numbers : R1-CEP 1999-179 - Rev 05
Status : Valid
Issue Date : 2017-03-29
Type : Chemical
Substance Number : 1203
Certificate Numbers : CEP 2020-208 - Rev 03
Status : Valid
Issue Date : 2025-08-29
Type : Chemical
Substance Number : 1203
Certificate Numbers : R2-CEP 1995-022 - Rev 05
Status : Valid
Issue Date : 2014-01-30
Type : Chemical
Substance Number : 140
Certificate Numbers : R1-CEP 2005-233 - Rev 03
Status : Valid
Issue Date : 2018-10-04
Type : Chemical
Substance Number : 1210
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registration Number : 303MF10070
Registrant's Address : Kloosterstraat 6, 5349 AB Oss, the Netherlands
Initial Date of Registration : 2021-04-22
Latest Date of Registration : 2021-04-22
Registration Number : 218MF10586
Registrant's Address : Kloosterstraat 6, 5349 AB Oss, the Netherlands
Initial Date of Registration : 2006-06-22
Latest Date of Registration : 2022-04-20
Registration Number : 218MF10506
Registrant's Address : Kloosterstraat 6, 5349 AB Oss, the Netherlands
Initial Date of Registration : 2006-05-15
Latest Date of Registration : 2024-11-13
Registration Number : 218MF10848
Registrant's Address : Kloosterstraat 6, 5349 AB Oss, the Netherlands
Initial Date of Registration : 2006-10-20
Latest Date of Registration : 2007-07-26
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Fine Chemicals Corporation PTY Ltd
Registration Number : 227MF10059
Registrant's Address : 15 Hawkins Avenue, Epping 1,7460, Cape Town, South Africa
Initial Date of Registration : 2015-03-06
Latest Date of Registration : 2024-03-13
Registration Number : 224MF10165
Registrant's Address : Kloosterstraat 6, 5349 AB Oss, the Netherlands
Initial Date of Registration : 2012-08-16
Latest Date of Registration : 2012-08-16
Registration Number : 219MF10237
Registrant's Address : Kloosterstraat 6, 5349 AB Oss, the Netherlands
Initial Date of Registration : 2007-07-17
Latest Date of Registration : 2020-01-31
Registration Number : 227MF10021
Registrant's Address : Kloosterstraat 6, 5349 AB Oss, the Netherlands
Initial Date of Registration : 2015-01-05
Latest Date of Registration : 2021-09-17
Registration Number : 227MF10199
Registrant's Address : Kloosterstraat 6, 5349 AB Oss, the Netherlands
Initial Date of Registration : 2015-08-06
Latest Date of Registration : 2020-10-01
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Fine Chemicals Corporation PTY Ltd
Registration Number : 306MF10066
Registrant's Address : 15 Hawkins Avenue, Epping 1,7460, Cape Town, South Africa
Initial Date of Registration : 2024-05-15
Latest Date of Registration : 2024-05-15
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : South Africa
Testosterone Propionate; Testosterone Phenylpropionate; Testosterone Isocaproate; Testosterone Decanoate
Dosage Form : Injection
Brand Name : SUSTANON 250
Dosage Strength : 30MG; 60MG; 60MG; 100M...
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : South Africa
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Services
Analytical
API Manufacturing
Pharma Service : API Manufacturing
Category : Clinical Supply
Sub Category : Overview
Pharma Service : API Manufacturing
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]API & Drug Product Development
Excipients
District Decision : Voluntary Action Indicated
Inspection End Date : 2025-04-04
Fine Chemicals Corporation PTY Ltd
City : Goodwood
State :
Country/Area : South Africa
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2025-04-04
District Decision : Official Action Indicated
Inspection End Date : 2024-09-17
City : Port Elizabeth
State :
Country/Area : South Africa
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Official Action Indicated
Inspection End Date : 2024-09-17
District Decision : Voluntary Action Indicated
Inspection End Date : 2022-11-24
City : Oss
State :
Country/Area : Netherlands
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2022-11-24
District Decision : Voluntary Action Indicated
Inspection End Date : 2022-11-24
City : Oss
State :
Country/Area : Netherlands
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2022-11-24
District Decision : Voluntary Action Indicated
Inspection End Date : 2021-04-16
City : Notre Dame de Bondeville
State :
Country/Area : France
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2021-04-16
District Decision : Voluntary Action Indicated
Inspection End Date : 2019-09-13
City : Oss
State :
Country/Area : Netherlands
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2019-09-13
District Decision : Voluntary Action Indicated
Inspection End Date : 2019-09-13
City : Oss
State :
Country/Area : Netherlands
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2019-09-13
District Decision : Voluntary Action Indicated
Inspection End Date : 2018-12-04
City : Port Elizabeth
State :
Country/Area : South Africa
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : Voluntary Action Indicated
Inspection End Date : 2018-12-04
District Decision : No Action Indicated
Inspection End Date : 2018-07-06
Fine Chemicals Corporation PTY Ltd
City : Goodwood
State :
Country/Area : South Africa
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2018-07-06
District Decision : No Action Indicated
Inspection End Date : 2018-05-31
City : Notre Dame de Bondeville
State :
Country/Area : France
Zip :
District :
Center :
Project Area : Drug Quality Assurance
District Decision : No Action Indicated
Inspection End Date : 2018-05-31
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
Aspen API is a supplier offers 63 products (APIs, Excipients or Intermediates).
Find a price of Desogestrel bulk with DMF, CEP, JDMF offered by Aspen API
Find a price of Estradiol bulk with DMF, CEP, JDMF offered by Aspen API
Find a price of Estriol bulk with DMF, CEP, JDMF offered by Aspen API
Find a price of Ethinyl Estradiol bulk with DMF, CEP, JDMF offered by Aspen API
Find a price of Leuprolide Acetate bulk with DMF, CEP, JDMF offered by Aspen API
Find a price of Mirtazapine bulk with DMF, CEP, JDMF offered by Aspen API
Find a price of Progesterone bulk with DMF, CEP, JDMF offered by Aspen API
Find a price of Rocuronium Bromide bulk with DMF, CEP, JDMF offered by Aspen API
Find a price of Azathioprine bulk with DMF, CEP offered by Aspen API
Find a price of Estradiol Valerate bulk with DMF, CEP offered by Aspen API
Find a price of Norethisterone bulk with DMF, CEP offered by Aspen API
Find a price of Norethisterone Acetate bulk with DMF, CEP offered by Aspen API
Find a price of Oxytocin bulk with DMF, CEP offered by Aspen API
Find a price of Ropivacaine Hydrochloride bulk with DMF, CEP offered by Aspen API
Find a price of Scopolamine bulk with DMF, CEP offered by Aspen API
Find a price of Testosterone bulk with DMF, CEP offered by Aspen API
Find a price of Baclofen bulk with DMF offered by Aspen API
Find a price of Benztropine bulk with DMF offered by Aspen API
Find a price of Conjugated Estrogens bulk with DMF offered by Aspen API
Find a price of Estriol bulk with CEP offered by Aspen API
Find a price of Ethynodiol Diacetate bulk with DMF offered by Aspen API
Find a price of Etonogestrel bulk with DMF offered by Aspen API
Find a price of Fentanyl bulk with CEP offered by Aspen API
Find a price of Fentanyl Citrate bulk with CEP offered by Aspen API
Find a price of Fluphenazine bulk with DMF offered by Aspen API
Find a price of Fluphenazine Decanoate bulk with DMF offered by Aspen API
Find a price of Ganirelix bulk with DMF offered by Aspen API
Find a price of Gonadorelin Acetate bulk with DMF offered by Aspen API
Find a price of Human Chorionic Gonadotropin bulk with DMF offered by Aspen API
Find a price of Leuprolide Acetate bulk with CEP offered by Aspen API
Find a price of Nandrolone Decanoate bulk with DMF offered by Aspen API
Find a price of Remifentanil bulk with DMF offered by Aspen API
Find a price of Ropivacaine Hydrochloride bulk with DMF offered by Aspen API
Find a price of Testosterone bulk with CEP offered by Aspen API
Find a price of Testosterone Cypionate bulk with DMF offered by Aspen API
Find a price of Testosterone Enanthate bulk with DMF offered by Aspen API
Find a price of Testosterone Undecanoate bulk with DMF offered by Aspen API
Find a price of Thiothixene bulk with DMF offered by Aspen API
Find a price of Tibolone bulk with CEP offered by Aspen API
Find a price of Vincristine Sulfate bulk with DMF offered by Aspen API
Find a price of Cidofovir bulk offered by Aspen API
Find a price of Clascoterone bulk offered by Aspen API
Find a price of Codeine bulk offered by Aspen API
Find a price of Codeine Phosphate bulk offered by Aspen API
Find a price of Conjugated Estrogens bulk offered by Aspen API
Find a price of Dihydrotestosterone bulk offered by Aspen API
Find a price of Estradiol bulk offered by Aspen API
Find a price of Fentanyl Hydrochloride bulk offered by Aspen API
Find a price of Goserelin Acetate bulk offered by Aspen API
Find a price of Hyoscine Butyl Bromide bulk offered by Aspen API
Find a price of Lanreotide Acetate bulk offered by Aspen API
Find a price of Leuprolide Acetate bulk offered by Aspen API
Find a price of Lynestrenol bulk offered by Aspen API
Find a price of Morphine Hydrochloride bulk offered by Aspen API
Find a price of Morphine Sulfate bulk offered by Aspen API
Find a price of Morphine Tartrate bulk offered by Aspen API
Find a price of Nusinersen bulk offered by Aspen API
Find a price of Pegcetacoplan bulk offered by Aspen API
Find a price of Segesterone Acetate bulk offered by Aspen API
Find a price of Semaglutide bulk offered by Aspen API
Find a price of Sugammadex Sodium bulk offered by Aspen API
Find a price of Testosterone Propionate bulk offered by Aspen API
Find a price of Vinblastine Sulfate bulk offered by Aspen API


 Aspen API
Aspen API