
Synopsis
0
KDMF
0
Australia
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Ocytocin
2. Pitocin
3. Syntocinon
1. 50-56-6
2. Pitocin
3. Endopituitrina
4. Ocytocin
5. Syntocinon
6. Orasthin
7. Oxytocinum
8. Oxitocina
9. Oxytocine
10. (1-hemicystine)oxytocin
11. Piton S
12. Alpha-hypophamine
13. 3-isoleucine-8-leucine Vasopressin
14. Oxytocic Hormone
15. Chebi:7872
16. Chembl395429
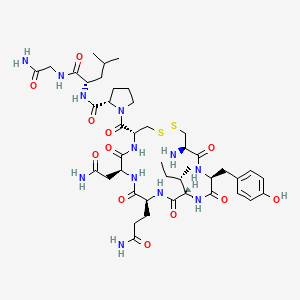
17. (2s)-1-[(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-[(2s)-butan-2-yl]-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[(2s)-1-[(2-amino-2-oxoethyl)amino]-4-methyl-1-oxopentan-2-yl]pyrrolidine-2-carboxamide
18. Oxystin
19. Partocon
20. Synthetic Oxytocin
21. Oxt
22. Ocytocinum
23. Ossitocina
24. Syntocinone
25. Oxetakain
26. Oxoject
27. Oxtocin
28. Presoxin
29. Synpitan
30. Syntocin
31. Utedrin
32. Uteracon
33. Di-sipidin
34. Nobitocin S
35. Atonin O
36. Oxytocin (syntocinon)
37. [3h]oxytocin
38. Oxetakain [czech]
39. 1-({(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-16-(4-hydroxybenzyl)-13-[(1s)-1-methylpropyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-4-yl}carbonyl)-l-prolyl-l-leucylglycinamide
40. L-cysteinyl-l-tyrosyl-l-isoleucyl-l-glutaminyl-l-asparaginyl-l-cysteinyl-l-prolyl-l-leucylglycinamide Cyclic(1-6)-disulfide
41. Ossitocina [dcit]
42. Oxytocin 5 Usp Units In Dextrose 5%
43. Oxytocin 10 Usp Units In Dextrose 5%
44. Oxytocin 20 Usp Units In Dextrose 5%
45. Oxytocine [inn-french]
46. Oxytocinum [inn-latin]
47. Oxitocina [inn-spanish]
48. Posterior Pituitary Extract
49. Unii-1jqs135eyn
50. A-hypophamine
51. Intertocine S
52. [3h]ot (human, Mouse, Rat)
53. Hsdb 2182
54. Oxytocin [usp:inn:ban:jan]
55. Syntocinon (tn)
56. Oxytocin (tn)
57. Oxytocin,(s)
58. (2s)-1-[(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-[(2s)-butan-2-yl]-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[(2s)-1-[(2-amino-2-oxoethyl)amino]-4-methyl-1-oxopentan-2-yl]pyrrolidine-2-carboxamide
59. Einecs 200-048-4
60. Pitocin (tn)
61. Mfcd00076731
62. Brn 3586108
63. Vasopressin, 3-l-isoleucine-8-l-leucine-
64. 1jqs135eyn
65. Schembl29048
66. Oxytocin (jp17/usp/inn)
67. Gtpl2174
68. Gtpl2176
69. Dtxsid8048361
70. Bcbcmap01_000094
71. Tnx1900
72. Tta-121
73. Amy25364
74. Cys-tyr-ile-gln-asn-cys-pro-leu-gly-nh2, Cyclic 1-6 Disulfide
75. Tnx-1900
76. Bdbm50205990
77. Ti-001
78. Akos015994657
79. Hs-2021
80. Ncgc00167132-01
81. Ac-28730
82. L-cysteinyl-l-tyrosyl-l-isoleucyl-l-glutaminyl-l-asparaginyl-l-cysteinyl-l-prolyl-l-leucylglycinamide Cyclic (1-6)-disulfide
83. C00746
84. D00089
85. 076o731
86. A828179
87. Q169960
88. Sr-01000945111
89. Sr-01000945111-1
90. W-105951
91. Oxytocin, European Pharmacopoeia (ep) Reference Standard
92. Oxytocin, United States Pharmacopeia (usp) Reference Standard
93. Oxytocin-(leucine-5,5,5-d3, Glycine-2,2-d2) Trifluoroacetate Salt
94. Oxytocin, Lyophilized Powder, ~15 Iu/mg Solid (prepared From Synthetic Oxytocin)
95. 1-[19-amino-7-(2-amino-2-oxo-ethyl)-10-(3-amino-3-oxo-propyl)-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-13-sec-butyl-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[1-[(2-amino-1-methyl-2-oxo-ethyl)carbamoyl]-3-methyl-butyl]pyrrolidine
96. L-cysteinyl-l-tyrosyl-l-isoleucyl-l-glutaminyl-l-asparaginyl-l-cysteinyl-l-prolyl-l-leucylglycinamide Cyclic (1-->6)-disulfide
97. Otx
| Molecular Weight | 1007.2 g/mol |
|---|---|
| Molecular Formula | C43H66N12O12S2 |
| XLogP3 | -2.6 |
| Hydrogen Bond Donor Count | 12 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 17 |
| Exact Mass | 1006.43645793 g/mol |
| Monoisotopic Mass | 1006.43645793 g/mol |
| Topological Polar Surface Area | 450 Ų |
| Heavy Atom Count | 69 |
| Formal Charge | 0 |
| Complexity | 1870 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Oxytocin |
| PubMed Health | Oxytocin |
| Drug Classes | Diagnostic Agent, Fetal Heart Rate Distress, Endocrine-Metabolic Agent, Uterine Stimulant |
| Drug Label | Pitocin (oxytocin injection, USP) is a sterile, clear, colorless aqueous solution of synthetic oxytocin, for intravenous infusion or intramuscular injection. Pitocin is a nonapeptide found in pituitary extracts from mammals. It is standardized to con... |
| Active Ingredient | Oxytocin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 300usp units/30ml (10usp units/ml); 10usp units/ml (10usp units/ml); 100usp units/10ml (10usp units/ml) |
| Market Status | Prescription |
| Company | Hikma Farmaceutica; Fresenius Kabi Usa; Hikma Maple |
| 2 of 4 | |
|---|---|
| Drug Name | Pitocin |
| PubMed Health | Oxytocin |
| Drug Classes | Diagnostic Agent, Fetal Heart Rate Distress, Endocrine-Metabolic Agent, Uterine Stimulant |
| Drug Label | Pitocin (oxytocin injection, USP) is a sterile, clear, colorless aqueous solution of synthetic oxytocin, for intravenous infusion or intramuscular injection. Pitocin is a nonapeptide found in pituitary extracts from mammals. It is standardized to con... |
| Active Ingredient | Oxytocin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10usp units/ml (10usp units/ml); 100usp units/10ml (10usp units/ml); 500usp units/50ml (10usp units/ml) |
| Market Status | Prescription |
| Company | Par Sterile Products |
| 3 of 4 | |
|---|---|
| Drug Name | Oxytocin |
| PubMed Health | Oxytocin |
| Drug Classes | Diagnostic Agent, Fetal Heart Rate Distress, Endocrine-Metabolic Agent, Uterine Stimulant |
| Drug Label | Pitocin (oxytocin injection, USP) is a sterile, clear, colorless aqueous solution of synthetic oxytocin, for intravenous infusion or intramuscular injection. Pitocin is a nonapeptide found in pituitary extracts from mammals. It is standardized to con... |
| Active Ingredient | Oxytocin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 300usp units/30ml (10usp units/ml); 10usp units/ml (10usp units/ml); 100usp units/10ml (10usp units/ml) |
| Market Status | Prescription |
| Company | Hikma Farmaceutica; Fresenius Kabi Usa; Hikma Maple |
| 4 of 4 | |
|---|---|
| Drug Name | Pitocin |
| PubMed Health | Oxytocin |
| Drug Classes | Diagnostic Agent, Fetal Heart Rate Distress, Endocrine-Metabolic Agent, Uterine Stimulant |
| Drug Label | Pitocin (oxytocin injection, USP) is a sterile, clear, colorless aqueous solution of synthetic oxytocin, for intravenous infusion or intramuscular injection. Pitocin is a nonapeptide found in pituitary extracts from mammals. It is standardized to con... |
| Active Ingredient | Oxytocin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10usp units/ml (10usp units/ml); 100usp units/10ml (10usp units/ml); 500usp units/50ml (10usp units/ml) |
| Market Status | Prescription |
| Company | Par Sterile Products |
Oxytocin is indicated for the medical rather than the elective induction of labor. Available data and information are inadequate to define the benefits-to-risks considerations in the use of the drug product for elective induction. Elective induction of labor is defined as the initiation of labor for convenience in an individual with a term pregnancy who is free of medical indications. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Oxytocin injection (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10243
Oxytocin is indicated for the initiation or improvement of uterine contractions, where this is desirable and considered suitable for reasons of fetal or maternal concern, in order to achieve early vaginal delivery. It is indicated for induction of labor in patients with a medical indication for the initiation of labor, such as Rh problems, maternal diabetes, preeclampsia at or near term, when delivery is in the best interests of mother and fetus or when membranes are prematurely ruptured and delivery is indicated... /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Oxytocin injection (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10243
Oxytocin is indicated for stimulation or reinforcement of labor, as in selected cases of uterine inertia... /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Oxytocin injection (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10243
Oxytocin is indicated as adjunctive therapy in the management of incomplete or inevitable abortion. In the first trimester, curettage is generally considered primary therapy. In second trimester abortion, oxytocin infusion will often be successful in emptying the uterus. Other means of therapy, however, may be required in such cases. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Oxytocin injection (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10243
For more Therapeutic Uses (Complete) data for Oxytocin (11 total), please visit the HSDB record page.
When oxytocin is administered in excessive dosage, with abortifacients or to sensitive patients, hyperstimulation of the uterus, with strong (hypertonic) and/or prolonged (tetanic) contractions, or a resting uterine tone of 15-20 mm H2O between contractions may occur, possibly resulting in uterine rupture, cervical and vaginal lacerations, postpartum hemorrhage, abruptio placentae, impaired uterine blood flow, amniotic fluid embolism, and fetal trauma including intracranial hemorrhage.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3348
Increased uterine motility may cause adverse fetal effects, including sinus bradycardia, tachycardia, premature ventricular complexes and other arrhythmias, permanent CNS or brain damage, and death secondary to asphyxia. Excessive maternal dosage or administration of the drug to sensitive women also can cause uteroplacental hypoperfusion and variable deceleration of fetal heart rate, fetal hypoxia, perinatal hepatic necrosis, and fetal hypercapnia. Rare incidents of pelvic hematoma have been reported, but these were probably also related to the high incidence of operative vaginal deliveries in primiparas, the fragility of engorged pelvic veins (especially if varicosed), and faulty episiotomy repair.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3348
When large amounts of oxytocin are administered, severe decreases in maternal systolic and diastolic blood pressure, increases in heart rate, systemic venous return and cardiac output, and arrhythmia may occur; these effects may be particularly hazardous to patients with valvular heart disease and those receiving spinal and epidural anesthesia.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3348
Postpartum bleeding may be increased by administration of oxytocin; this effect may be related to reports of oxytocin-induced thrombocytopenia, afibrinogenemia, and hypoprothrombinemia. By carefully controlling delivery, the incidence of postpartum bleeding may be minimized.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3348
For more Drug Warnings (Complete) data for Oxytocin (22 total), please visit the HSDB record page.
Administration of exogenous oxytocin is indicated in the antepartum period to initiate or improve uterine contractions for vaginal delivery in situations where there is fetal or maternal concern. For example, It may be used to induce labor in cases of Rh sensitization, maternal diabetes, preeclampsia at or near term, and when delivery is indicated due to prematurely ruptured membranes. Importantly, oxytocin is not approved or indicated for elective induction of labor. Oxytocin may be used to reinforce labor in select cases of uterine inertia and as adjunctive therapy in the management of incomplete or inevitable abortion. In the postpartum period, oxytocin may be used to induced contractions in the 3rd stage of labor and to control postpartum bleeding or hemorrhage.
Oxytocin is a nonapeptide, pleiotropic hormone that exerts important physiological effects. It is most well known to stimulate parturition and lactation, but also has important physiological influences on metabolic and cardiovascular functions, sexual and maternal behaviour, pair bonding, social cognition, and fear conditioning. It is worth noting that oxytocin receptors are not limited to the reproductive system but can be found in many peripheral tissues and in central nervous system structures including the brain stem and amygdala.
Oxytocics
Drugs that stimulate contraction of the myometrium. They are used to induce LABOR, OBSTETRIC at term, to prevent or control postpartum or postabortion hemorrhage, and to assess fetal status in high risk pregnancies. They may also be used alone or with other drugs to induce abortions (ABORTIFACIENTS). Oxytocics used clinically include the neurohypophyseal hormone OXYTOCIN and certain prostaglandins and ergot alkaloids. (From AMA Drug Evaluations, 1994, p1157) (See all compounds classified as Oxytocics.)
H - Systemic hormonal preparations, excl. sex hormones and insulins
H01 - Pituitary and hypothalamic hormones and analogues
H01B - Posterior pituitary lobe hormones
H01BB - Oxytocin and analogues
H01BB02 - Oxytocin
Absorption
Oxytocin is administered parenterally and is fully bioavailable. It takes approximately 40 minutes for oxytocin to reach steady-state concentrations in the plasma after parenteral administration.
Route of Elimination
The enzyme oxytocinase is largely responsible for the metabolism and regulation of oxytocin levels in pregnancy; only a small percentage of the neurohormone is excreted in the urine unchanged.
Clearance
In a study that observed 10 women who were given oxytocin to induce labor, the mean metabolic clearance rate was 7.87 mL/min.
Oxytocin is destroyed by chymotrypsin in the GI tract. Uterine response occurs almost immediately and subsides within 1 hour following iv administration of oxytocin. Following im injection of the drug, uterine response occurs within 3-5 minutes and persists for 2-3 hours. Following intranasal application of 10-20 units of oxytocin (nasal preparations are no longer commercially available in the US), contractions of myoepithelial tissue surrounding the alveoli of the breasts begin within a few minutes and continue for 20 minutes; iv oxytocin produces the same effect with a dose of 100-200 milliunits.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3349
Like vasopressin, oxytocin is distributed throughout the extracellular fluid. Small amounts of oxytocin probably reach the fetal circulation.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3349
It is not known whether this drug is excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for Oxytocin injection (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10243
Its rapid removal from plasma is accomplished largely by the kidney and the liver. Only small amounts oxytocin are excreted in the urine unchanged.
US Natl Inst Health; DailyMed. Current Medication Information for Oxytocin injection (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10243
For more Absorption, Distribution and Excretion (Complete) data for Oxytocin (7 total), please visit the HSDB record page.
Oxytocin is rapidly removed from the plasma by the liver and kidney. The enzyme oxytocinase is largely responsible for the metabolism and regulation of oxytocin levels in pregnancy and only a small percentage of the neurohormone is excreted in the urine unchanged. Oxytocinase activity increases throughout pregnancy and peaks in the plasma, placenta and uterus near term. The placenta is a key source of oxytocinase during gestation and produces increasing amounts of the enzyme in response to increasing levels of oxytocin produced by the mother. Oxytocinase activity is also expressed in mammary glands, heart, kidney, and the small intestine. Lower levels of activity can be found in the brain, spleen, liver, skeletal muscle, testes, and colon. The level of oxytocin degradation is negligible in non-pregnant women, men, and cord blood.
Oxytocinase, a circulating enzyme produced early in pregnancy, is also capable of inactivating the polypeptide.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3349
During pregnancy ... "oxytocinase" ... is capable of inactivating oxytocin by cleavage of the 1-cysteine to 2-tyrosine peptide bond.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 938
The plasma half-life of oxytocin ranges from 1-6 minutes. The half-life is decreased in late pregnancy and during lactation.
Oxytocin has a plasma half-life of about 3 to 5 minutes.
US Natl Inst Health; DailyMed. Current Medication Information for Oxytocin injection (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10243
Oxytocin plays a vital role in labour and delivery. The hormone is produced in the hypothalamus and is secreted from the paraventricular nucleus to the posterior pituitary where it is stored. It is then released in pulses during childbirth to induce uterine contractions. The concentration of oxytocin receptors on the myometrium increases significantly during pregnancy and reaches a peak in early labor. Activation of oxytocin receptors on the myometrium triggers a downstream cascade that leads to increased intracellular calcium in uterine myofibrils which strengthens and increases the frequency of uterine contractions. In humans, most hormones are regulated by negative feedback; however, oxytocin is one of the few that is regulated by positive feedback. The head of the fetus pushing on the cervix signals the release of oxytocin from the posterior pituitary of the mother. Oxytocin then travels to the uterus where it stimulates uterine contractions. The elicited uterine contractions will then stimulate the release of increasing amounts of oxytocin. This positive feedback loop will continue until parturition. Since exogenously administered and endogenously secreted oxytocin result in the same effects on the female reproductive system, synthetic oxytocin may be used in specific instances during the antepartum and postpartum period to induce or improve uterine contractions.
The pharmacologic and clinical properties of oxytocin are identical with those of naturally occurring oxytocin principle of the posterior lobe of pituitary. Oxytocin exerts a selective action on the smooth musculature of the uterus, particularly toward the end of pregnancy, during labor, and immediately following delivery. Oxytocin stimulates rhythmic contractions of the uterus, increases the frequency of existing contractions, and raises the tone of the uterine musculature.
US Natl Inst Health; DailyMed. Current Medication Information for Oxytocin injection (June 2009). Available from, as of February 10, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10243
Oxytocin indirectly stimulates contraction of uterine smooth muscle by increasing the sodium permeability of uterine myofibrils. High estrogen concentrations lower the threshold for uterine response to oxytocin. Uterine response to oxytocin increases with the duration of pregnancy and is greater in patients who are in labor than those not in labor; only very large doses elicit contractions in early pregnancy. Contractions produced in the term uterus by oxytocin are similar to those occurring during spontaneous labor. In the term uterus, oxytocin increases the amplitude and frequency of uterine contractions which in turn tend to decrease cervical activity producing dilation and effacement of the cervix and to transiently impede uterine blood flow.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3348
Oxytocin contracts myoepithelial cells surrounding the alveoli of the breasts, forcing milk from the alveoli into the larger ducts and thus facilitating milk ejection. The drug possesses no galactopoietic properties.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3348
Oxytocin produces vasodilation of vascular smooth muscle, increasing renal, coronary, and cerebral blood flow. Blood pressure is usually unchanged, but following iv administration of very large doses or undiluted solutions, blood pressure may decrease transiently, and tachycardia and an increase in cardiac output may be reflexly induced. Any initial fall in blood pressure is usually followed by a small but sustained increase in blood pressure. In contrast to vasopressin, oxytocin has minimal antidiuretic effects; however, water intoxication may occur when oxytocin is administered with an excessive volume of electrolyte-free iv fluids and/or at too rapid a rate.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3349
For more Mechanism of Action (Complete) data for Oxytocin (15 total), please visit the HSDB record page.
Registration Number : 222MF10157
Registrant's Address : 20901 Higgins Court Torrance, California USA 90501-1722
Initial Date of Registration : 2010-05-24
Latest Date of Registration : 2010-05-24

Registration Number : 226MF10086
Registrant's Address : 20 Kelly Court, Menlo Park, CA 94025 USA
Initial Date of Registration : 2014-05-09
Latest Date of Registration : 2023-09-20

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
24
PharmaCompass offers a list of Oxytocin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Oxytocin manufacturer or Oxytocin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Oxytocin manufacturer or Oxytocin supplier.
PharmaCompass also assists you with knowing the Oxytocin API Price utilized in the formulation of products. Oxytocin API Price is not always fixed or binding as the Oxytocin Price is obtained through a variety of data sources. The Oxytocin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Oxytocin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Oxytocin, including repackagers and relabelers. The FDA regulates Oxytocin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Oxytocin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Oxytocin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Oxytocin supplier is an individual or a company that provides Oxytocin active pharmaceutical ingredient (API) or Oxytocin finished formulations upon request. The Oxytocin suppliers may include Oxytocin API manufacturers, exporters, distributors and traders.
click here to find a list of Oxytocin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Oxytocin DMF (Drug Master File) is a document detailing the whole manufacturing process of Oxytocin active pharmaceutical ingredient (API) in detail. Different forms of Oxytocin DMFs exist exist since differing nations have different regulations, such as Oxytocin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Oxytocin DMF submitted to regulatory agencies in the US is known as a USDMF. Oxytocin USDMF includes data on Oxytocin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Oxytocin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Oxytocin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Oxytocin Drug Master File in Japan (Oxytocin JDMF) empowers Oxytocin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Oxytocin JDMF during the approval evaluation for pharmaceutical products. At the time of Oxytocin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Oxytocin suppliers with JDMF on PharmaCompass.
A Oxytocin CEP of the European Pharmacopoeia monograph is often referred to as a Oxytocin Certificate of Suitability (COS). The purpose of a Oxytocin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Oxytocin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Oxytocin to their clients by showing that a Oxytocin CEP has been issued for it. The manufacturer submits a Oxytocin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Oxytocin CEP holder for the record. Additionally, the data presented in the Oxytocin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Oxytocin DMF.
A Oxytocin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Oxytocin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Oxytocin suppliers with CEP (COS) on PharmaCompass.
A Oxytocin written confirmation (Oxytocin WC) is an official document issued by a regulatory agency to a Oxytocin manufacturer, verifying that the manufacturing facility of a Oxytocin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Oxytocin APIs or Oxytocin finished pharmaceutical products to another nation, regulatory agencies frequently require a Oxytocin WC (written confirmation) as part of the regulatory process.
click here to find a list of Oxytocin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Oxytocin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Oxytocin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Oxytocin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Oxytocin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Oxytocin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Oxytocin suppliers with NDC on PharmaCompass.
Oxytocin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Oxytocin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Oxytocin GMP manufacturer or Oxytocin GMP API supplier for your needs.
A Oxytocin CoA (Certificate of Analysis) is a formal document that attests to Oxytocin's compliance with Oxytocin specifications and serves as a tool for batch-level quality control.
Oxytocin CoA mostly includes findings from lab analyses of a specific batch. For each Oxytocin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Oxytocin may be tested according to a variety of international standards, such as European Pharmacopoeia (Oxytocin EP), Oxytocin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Oxytocin USP).

