
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Australia
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
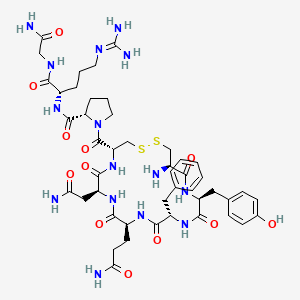

1. Arg Vasopressin
2. Arg-vasopressin
3. Arginine Vasopressin
4. Argipressin Tannate
5. Vasopressin, Arginine
1. Arginine Vasopressin
2. 113-79-1
3. Argipressine
4. Pitressin
5. Beta-hypophamine
6. 8-arginine-vasopressin
7. Argipressin Tannate
8. Vasopressin (arginine Form)
9. Arginine-vasopressin
10. Chebi:34543
11. Vasophysin
12. Arg-vasopressin
13. Chembl373742
14. (arg8)-vasopressin
15. [arg8]-vasopressin
16. (2s)-1-[(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[(2s)-1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]pyrrolidine-2-carboxamide
17. 8-l-arginine Vasopressin
18. Argipresina
19. Argipressina
20. Argipressinum
21. Avp
22. Arg8-vasopressin
23. Argipressin [inn]
24. Argipressina [dcit]
25. Ncgc00166306-01
26. Argipressin [inn:ban]
27. 8-l-arginine-vasopressin
28. Argipresina [inn-spanish]
29. Argipressine [inn-french]
30. Argipressinum [inn-latin]
31. Unii-y4907o6mfd
32. Rindervasopressin
33. [3h]vasopressin
34. Einecs 204-035-4
35. 3-(phenylalanine)-8-arginineoxytocin
36. Arg8-vasopressin;avp
37. Arginine-8-vasopressin
38. Argipressin Or Lypressin
39. [8-arginine]vasopressin
40. [3h]argipressin Tannate
41. Antidiuretic Hormone (adh)
42. Arginine Vasopressin (avp)
43. Argipressin Tannate [usan]
44. Arginine Antidiuretic Hormone
45. Dsstox_cid_28324
46. Dsstox_rid_82752
47. Dsstox_gsid_48349
48. Schembl43139
49. Vasopressin, 8-l-arginine-
50. [cyclo S-s]cyfqncprg-nh2
51. Gtpl2168
52. Y4907o6mfd
53. Dtxsid0048349
54. Schembl17874853
55. Bdbm35667
56. Tox21_113037
57. Bdbm50044777
58. Mfcd00076738
59. Ncgc00166306-02
60. Ncgc00188439-01
61. Cas-113-79-1
62. H-cys-tyr-phe-gln-asn-cys-pro-arg-gly-nh2
63. Oxytocin, 3-(l-phenylalanine)-8-l-arginine-
64. Vasopressin, 8-l-arginine- (7ci,8ci,9ci)
65. Roxybenzyl-6,9,12,15,18-pentaoxo- (6ci)
66. 113a791
67. Q183011
68. Cys-tyr-phe-gln-asn-cys-pro-arg-gly-nh2[disulfide Bridge: 1-6]
69. (2s)-1-[(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-13-(phenylmethyl)1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[(2s)-1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]pyrrolidine-2-carboxamide
70. 1,2-dithia-5,8,11,14,17-pentaazacycloeicosane-10-propionamide, 19-amino-13-benzyl-7-(carbamoylmethyl)-4-[2-[[1-[(carbamoylmethyl)carbamoyl]-4-guanidinobutyl]carbamoyl]-1-pyrrolidinylcarbonyl]-16-p-hyd
71. 1-{[(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-(4-hydroxybenzyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-4-yl]carbonyl}-l-prolyl-l-arginylglycinamide
72. Glycinamide, L-cysteinyl-l-tyrosyl-l-phenylalanyl-l-glutaminyl-l-asparaginyl-l-cysteinyl-l-prolyl-l-arginyl-, Cyclic (1>6)-disulfide
| Molecular Weight | 1084.2 g/mol |
|---|---|
| Molecular Formula | C46H65N15O12S2 |
| XLogP3 | -4.8 |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 19 |
| Exact Mass | 1083.43785492 g/mol |
| Monoisotopic Mass | 1083.43785492 g/mol |
| Topological Polar Surface Area | 515 Ų |
| Heavy Atom Count | 75 |
| Formal Charge | 0 |
| Complexity | 2070 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
Antidiuretic Agents
Agents that reduce the excretion of URINE, most notably the octapeptide VASOPRESSINS. (See all compounds classified as Antidiuretic Agents.)
Hemostatics
Agents acting to arrest the flow of blood. Absorbable hemostatics arrest bleeding either by the formation of an artificial clot or by providing a mechanical matrix that facilitates clotting when applied directly to the bleeding surface. These agents function more at the capillary level and are not effective at stemming arterial or venous bleeding under any significant intravascular pressure. (See all compounds classified as Hemostatics.)
About the Company : HRV Pharma is a global manufacturer, seller, and exporter of APIs, intermediates, pellets, food-grade chemicals, food additives, and food ingredients. The company provides sourcing...
About the Company : Zeon Pharma Industries India Pvt. Ltd. is an ISO 9001:2015, cGMP, and WHO-GMP certified company with a dedicated manufacturing facility for Bulk Drugs (APIs), phytochemicals, herba...
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
About the Company : Omgene Life Sciences Pvt. Ltd. is an R&D-driven biopharmaceutical company specializing in biopharmaceuticals, peptides, semi-synthetic, and synthetic actives. As a vertically integ...
About the Company : Apicore LLC, a wholly owned subsidiary of RK Pharma Inc is a leading process R&D and API manufacturing service provider for the worldwide pharmaceutical industry. We offer a wide p...

About the Company : Founded in 1986 by Mr. P.V. Ramaprasad Reddy, Mr. K. Nityananda Reddy and a small group of highly committed professionals, Aurobindo Pharma was born off a vision. The company comme...

About the Company : JIN DUN Medical Research Institute is affiliated to Shanghai JIN DUN Industrial Co., Ltd., headquartered in Shanghai, adjacent to Hongqiao High-speed Railway Station and Hongqiao I...

About the Company : Consistent growth and sustainability is a multidimensional aspiration for all at Macleods, we remained focused on providing quality and affordable medicines to billions of ailing p...

About the Company : Piramal Pharma Solutions (PPS) is a CDMO that provides end-to-end solutions for drug development and manufacturing across the drug life cycle to its clients in North America, Europ...

About the Company : PolyPeptide is a Contract Development & Manufacturing Organization (CDMO) focusing on proprietary and generic GMP-grade peptides used by pharmaceutical and biotech companies in app...

About the Company : Shenzhen JYMed Technology Co.,Ltd is a high-tech enterprise engaged in research and development, manufacturing and commercialization of peptides based products, including active ph...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2024-05-08
Application Number : 213988
RX/OTC/DISCN : RX
RLD : No
TE Code : AP
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : VASOPRESSIN
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 200UNITS/10ML(20UNITS/ML)
Approval Date :
Application Number : 212945
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2022-08-05
Application Number : 212944
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/100ML (0.2UNITS/ML)
Approval Date : 2023-12-06
Application Number : 217987
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2023-05-26
Application Number : 213206
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : VASOPRESSIN IN SODIUM CHLORIDE 0.9%
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/100ML (0.2UNITS/ML)
Approval Date : 2024-07-11
Application Number : 217766
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : VASOPRESSIN IN SODIUM CHLORIDE 0.9%
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 40UNITS/100ML (0.4UNITS/ML)
Approval Date : 2024-07-11
Application Number : 217766
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : VASOPRESSIN IN SODIUM CHLORIDE 0.9%
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 50UNITS/50ML (1UNIT/ML)
Approval Date : 2024-07-11
Application Number : 217766
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

Brand Name : VASOSTRICT
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 40UNITS/100ML (0.4UNITS/ML)
Approval Date : 2020-04-15
Application Number : 204485
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AP

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Brand Name : VASOSTRICT
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 60UNITS/100ML (0.6UNITS/ML)
Approval Date : 2020-04-15
Application Number : 204485
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
52
PharmaCompass offers a list of Arginine Vasopressin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Arginine Vasopressin manufacturer or Arginine Vasopressin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Arginine Vasopressin manufacturer or Arginine Vasopressin supplier.
PharmaCompass also assists you with knowing the Arginine Vasopressin API Price utilized in the formulation of products. Arginine Vasopressin API Price is not always fixed or binding as the Arginine Vasopressin Price is obtained through a variety of data sources. The Arginine Vasopressin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Arginine Vasopressin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Arginine Vasopressin, including repackagers and relabelers. The FDA regulates Arginine Vasopressin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Arginine Vasopressin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Arginine Vasopressin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Arginine Vasopressin supplier is an individual or a company that provides Arginine Vasopressin active pharmaceutical ingredient (API) or Arginine Vasopressin finished formulations upon request. The Arginine Vasopressin suppliers may include Arginine Vasopressin API manufacturers, exporters, distributors and traders.
click here to find a list of Arginine Vasopressin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Arginine Vasopressin DMF (Drug Master File) is a document detailing the whole manufacturing process of Arginine Vasopressin active pharmaceutical ingredient (API) in detail. Different forms of Arginine Vasopressin DMFs exist exist since differing nations have different regulations, such as Arginine Vasopressin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Arginine Vasopressin DMF submitted to regulatory agencies in the US is known as a USDMF. Arginine Vasopressin USDMF includes data on Arginine Vasopressin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Arginine Vasopressin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Arginine Vasopressin suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Arginine Vasopressin Drug Master File in Korea (Arginine Vasopressin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Arginine Vasopressin. The MFDS reviews the Arginine Vasopressin KDMF as part of the drug registration process and uses the information provided in the Arginine Vasopressin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Arginine Vasopressin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Arginine Vasopressin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Arginine Vasopressin suppliers with KDMF on PharmaCompass.
A Arginine Vasopressin written confirmation (Arginine Vasopressin WC) is an official document issued by a regulatory agency to a Arginine Vasopressin manufacturer, verifying that the manufacturing facility of a Arginine Vasopressin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Arginine Vasopressin APIs or Arginine Vasopressin finished pharmaceutical products to another nation, regulatory agencies frequently require a Arginine Vasopressin WC (written confirmation) as part of the regulatory process.
click here to find a list of Arginine Vasopressin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Arginine Vasopressin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Arginine Vasopressin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Arginine Vasopressin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Arginine Vasopressin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Arginine Vasopressin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Arginine Vasopressin suppliers with NDC on PharmaCompass.
Arginine Vasopressin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Arginine Vasopressin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Arginine Vasopressin GMP manufacturer or Arginine Vasopressin GMP API supplier for your needs.
A Arginine Vasopressin CoA (Certificate of Analysis) is a formal document that attests to Arginine Vasopressin's compliance with Arginine Vasopressin specifications and serves as a tool for batch-level quality control.
Arginine Vasopressin CoA mostly includes findings from lab analyses of a specific batch. For each Arginine Vasopressin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Arginine Vasopressin may be tested according to a variety of international standards, such as European Pharmacopoeia (Arginine Vasopressin EP), Arginine Vasopressin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Arginine Vasopressin USP).

