
Synopsis
Synopsis
0
Australia
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
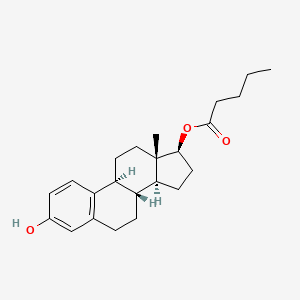

1. 17 Beta Estradiol
2. 17 Beta Oestradiol
3. 17 Beta-estradiol
4. 17 Beta-oestradiol
5. Aerodiol
6. Delestrogen
7. Estrace
8. Estraderm Tts
9. Estradiol
10. Estradiol 17 Alpha
11. Estradiol 17 Beta
12. Estradiol 17beta
13. Estradiol Anhydrous
14. Estradiol Hemihydrate
15. Estradiol Hemihydrate, (17 Alpha)-isomer
16. Estradiol Monohydrate
17. Estradiol Valeriante
18. Estradiol, (+-)-isomer
19. Estradiol, (-)-isomer
20. Estradiol, (16 Alpha,17 Alpha)-isomer
21. Estradiol, (16 Alpha,17 Beta)-isomer
22. Estradiol, (17-alpha)-isomer
23. Estradiol, (8 Alpha,17 Beta)-(+-)-isomer
24. Estradiol, (8 Alpha,17 Beta)-isomer
25. Estradiol, (9 Beta,17 Alpha)-isomer
26. Estradiol, (9 Beta,17 Beta)-isomer
27. Estradiol, Monosodium Salt
28. Estradiol, Sodium Salt
29. Estradiol-17 Alpha
30. Estradiol-17 Beta
31. Estradiol-17beta
32. Oestradiol
33. Ovocyclin
34. Progynon Depot
35. Progynon-depot
36. Progynova
37. Vivelle
1. 979-32-8
2. Estradiol 17-valerate
3. Delestrogen
4. Estradiol Valerianate
5. Oestradiol Valerate
6. Estraval
7. Neofollin
8. Progynova
9. Femogex
10. Progynon-depot
11. Atladiol
12. Climaval
13. Deladiol
14. Duratrad
15. Estate
16. Dura-estradiol
17. Exten Strone
18. Depo Estro Med
19. Repo-estra
20. Delestrogen 4x
21. Estraval Pa
22. Estraval 2x
23. Estroval-10
24. Delahormone Unimatic
25. Pelanin
26. Depo-estro-med
27. Femogen-l.a.
28. Gynogen L.a. 20
29. Gynogen L.a. 40
30. Deladumone
31. Estradiol, 17-valerate
32. Nsc-17590
33. Component Of Ditate
34. 17-beta-estradiol 17-valerate
35. B-estradiol 17-valerate
36. Component Of Deluteval 2x
37. Estradiol 17.beta.-valerate
38. Component Of Mal-o-fem L.a
39. .beta.-estradiol 17-valerate
40. Mls000028449
41. Chebi:31561
42. Okg364o896
43. 3-hydroxy-17beta-valeroyloxyestra-1,3,5(10)-trien
44. [(8r,9s,13s,14s,17s)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] Pentanoate
45. Cyclocur
46. Gynokadin
47. Merimono
48. Nuvelle
49. Postoval
50. Primogyna
51. Ronfase
52. Smr000058346
53. Estradiol Depot
54. Primogyn-depot
55. Beta-estradiol 17-valerate
56. 17-pentanoyl-estra-1,3,5(10)-triene-3,17beta-diol
57. Gynogen L.a. 10
58. (17beta)-3-hydroxyestra-1,3,5(10)-trien-17-yl Valerate
59. Valergen
60. Estradiol 17beta-valerate
61. Estradiol Valerate (van)
62. Mfcd00056541
63. Estradioli Valeras
64. Valerate D'estradiol
65. Ccris 5571
66. Valerato De Estradiol
67. Estradioli Valeras [inn-latin]
68. Oestradiol-17b-valerate
69. Valerate D'estradiol [inn-french]
70. Einecs 213-559-2
71. Nsc 17590
72. Valerato De Estradiol [inn-spanish]
73. Altadiol
74. Pelanin Depot
75. Unii-okg364o896
76. Estradiol-valerate
77. Gynogen La
78. Deladumone (tn)
79. Ncgc00094673-01
80. (8r,9s,13s,14s,17s)-3-hydroxy-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6h-cyclopenta[a]phenanthren-17-yl Pentanoate
81. 3-hydroxy-17beta-valeroyloxyestra-1,3,5(10)-triene
82. Gynogen L.a.10
83. Estradiol Valerate [usp:inn:ban:jan]
84. St075188
85. Estradiol Valerate Icrs
86. Opera_id_444
87. Estradiol 17?-valerate
88. Dsstox_cid_3004
89. Chembl1511
90. Dsstox_rid_76827
91. Dsstox_gsid_23004
92. Schembl40563
93. Mls001146931
94. Gtpl7655
95. Dtxsid8023004
96. Estradiol Valerate [inn]
97. Estradiol Valerate [jan]
98. Estradiol Valerate [vandf]
99. Hms2232l17
100. Hms3259c12
101. Hms3715b21
102. Estradiol Valerate [mart.]
103. Bcp11918
104. Estra-1,3,5(10)-triene-3,17-diol (17-beta)-, 17-pentanoate
105. Estra-1,3,5(10)-triene-3,17-diol(17beta)-, 17-pentanoate
106. Estradiol Valerate (jan/usp/inn)
107. Estradiol Valerate [usp-rs]
108. Estradiol Valerate [who-dd]
109. Hy-b0672
110. Nsc17590
111. Zinc3881556
112. Estradiol 17-valerate [mi]
113. Tox21_111312
114. Estra-1,3,5(10)-triene-3,17-diol (17.beta.)-, 17-pentanoate
115. Lmst02010039
116. S3149
117. Akos005267158
118. Estradiol-17-valerate; 1,3,5(10)-estratriene-3,17b-diol 17-pentanote
119. Ccg-221130
120. Db13956
121. Estradiol Valerate [green Book]
122. Fd12049
123. Ks-5213
124. Nc00560
125. Estradiol Valerate [orange Book]
126. Estradiol Valerate [ep Monograph]
127. Estradiol Valerate For System Suitability
128. Ncgc00018166-02
129. Ncgc00018166-14
130. (1s,11s,14s,15s,10r)-5-hydroxy-15-methyltetracyclo[8.7.0.0<2,7>.0<11,15>]hepta Deca-2,4,6-trien-14-yl Pentanoate
131. Cas-979-32-8
132. Cpd000058346
133. Estradiol Valerate [usp Monograph]
134. B1506
135. Ditate-ds Component Estradiol Valerate
136. E0876
137. D01413
138. Ab00382980_15
139. Estradiol Valerate Component Of Ditate-ds
140. 979e328
141. A845765
142. Sr-01000003061
143. Q-201506
144. Q5401768
145. Sr-01000003061-3
146. 1,3,5(10)-estratrien-3,17beta-diol 17-valerate
147. Brd-k66766661-001-19-2
148. (17.beta.)-estra-1,3,5(10)-triene-3,17-diol 17-valerate
149. (17beta)-3-hydroxyestra-1(10),2,4-trien-17-yl Pentanoate
150. Estra-1,5(10)-triene-3,17-diol (17.beta.)-, 17-pentanoate
151. Estra-1,3,5(10)-triene-3,17-diol(17.beta.)-, 17-pentanoate
152. [(13s,17s)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] Pentanoate
153. [(8r,9s,13s,14s,17s)-13-methyl-3-oxidanyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] Pentanoate
154. Pentanoic Acid [(8r,9s,13s,14s,17s)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] Ester
| Molecular Weight | 356.5 g/mol |
|---|---|
| Molecular Formula | C23H32O3 |
| XLogP3 | 6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 356.23514488 g/mol |
| Monoisotopic Mass | 356.23514488 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 518 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Delestrogen |
| PubMed Health | Estradiol (Injection) |
| Drug Classes | Endocrine-Metabolic Agent, Musculoskeletal Agent |
| Drug Label | DELESTROGEN (estradiol valerate injection, USP) contains estradiol valerate, a long-acting estrogen in sterile oil solutions for intramuscular use. These solutions are clear, colorless to pale yellow. Formulations (per mL): 10 mg estradiol valerate... |
| Active Ingredient | Estradiol valerate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 20mg/ml; 40mg/ml; 10mg/ml |
| Market Status | Prescription |
| Company | Par Sterile Products |
| 2 of 4 | |
|---|---|
| Drug Name | Estradiol valerate |
| Drug Label | Estradiol valerate injection, USP contains estradiol valerate, a long-acting estrogen in sterile oil solutions for intramuscular use. These solutions are clear, colorless to pale yellow. Formulations (per mL): 10 mg estradiol valerate in a vehicle co... |
| Active Ingredient | Estradiol valerate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 20mg/ml; 40mg/ml; 10mg/ml |
| Market Status | Prescription |
| Company | Luitpold; Sandoz |
| 3 of 4 | |
|---|---|
| Drug Name | Delestrogen |
| PubMed Health | Estradiol (Injection) |
| Drug Classes | Endocrine-Metabolic Agent, Musculoskeletal Agent |
| Drug Label | DELESTROGEN (estradiol valerate injection, USP) contains estradiol valerate, a long-acting estrogen in sterile oil solutions for intramuscular use. These solutions are clear, colorless to pale yellow. Formulations (per mL): 10 mg estradiol valerate... |
| Active Ingredient | Estradiol valerate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 20mg/ml; 40mg/ml; 10mg/ml |
| Market Status | Prescription |
| Company | Par Sterile Products |
| 4 of 4 | |
|---|---|
| Drug Name | Estradiol valerate |
| Drug Label | Estradiol valerate injection, USP contains estradiol valerate, a long-acting estrogen in sterile oil solutions for intramuscular use. These solutions are clear, colorless to pale yellow. Formulations (per mL): 10 mg estradiol valerate in a vehicle co... |
| Active Ingredient | Estradiol valerate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 20mg/ml; 40mg/ml; 10mg/ml |
| Market Status | Prescription |
| Company | Luitpold; Sandoz |
Estradiol valerate is commercially available as an intramuscular injection as the product Delestrogen and is indicated for the treatment of moderate to severe vasomotor symptoms and vulvovaginal atrophy due to menopause, for the treatment of hypoestrogenism due to hypogonadism, castration or primary ovarian failure, and for the treatment of advanced androgen-dependent carcinoma of the prostate (for palliation only). Estradiol valerate is also available in combination with [DB09123] as the commercially available product Natazia used for the prevention of pregnancy and for the treatment of heavy menstrual bleeding.
FDA Label
Estrogen mediates its effects across the body through potent agonism of the Estrogen Receptor (ER), which is located in various tissues including in the breasts, uterus, ovaries, skin, prostate, bone, fat, and brain. Estradiol binds to both subtypes of the Estrogen Receptor: Estrogen Receptor Alpha (ER) and Estrogen Receptor Beta (ER). Estradiol also acts as a potent agonist of G Protein-coupled Estrogen Receptor (GPER), which has recently been recognized as a major mediator of estradiol's rapid cellular effects.
Estrogens
Compounds that interact with ESTROGEN RECEPTORS in target tissues to bring about the effects similar to those of ESTRADIOL. Estrogens stimulate the female reproductive organs, and the development of secondary female SEX CHARACTERISTICS. Estrogenic chemicals include natural, synthetic, steroidal, or non-steroidal compounds. (See all compounds classified as Estrogens.)
Absorption
IM Injection: When conjugated with aryl and alkyl groups for parenteral administration, the rate of absorption of oily preparations is slowed with a prolonged duration of action, such that a single intramuscular injection of estradiol valerate or estradiol cypionate is absorbed over several weeks. Natazia: After oral administration of estradiol valerate, cleavage to 17-estradiol and valeric acid takes place during absorption by the intestinal mucosa or in the course of the first liver passage. This gives rise to estradiol and its metabolites, estrone and other metabolites. Maximum serum estradiol concentrations of 73.3 pg/mL are reached at a median of approximately 6 hours (range: 1.512 hours) and the area under the estradiol concentration curve [AUC(024h)] was 1301 pgh/mL after single ingestion of a tablet containing 3 mg estradiol valerate under fasted condition on Day 1 of the 28-day sequential regimen.
Route of Elimination
Estradiol, estrone and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
Exogenous estrogens are metabolized using the same mechanism as endogenous estrogens. Estrogens are partially metabolized by cytochrome P450.
Estradiol enters target cells freely (e.g., female organs, breasts, hypothalamus, pituitary) and interacts with a target cell receptor. When the estrogen receptor has bound its ligand it can enter the nucleus of the target cell, and regulate gene transcription which leads to formation of messenger RNA. The mRNA interacts with ribosomes to produce specific proteins that express the effect of estradiol upon the target cell. Estrogens increase the hepatic synthesis of sex hormone binding globulin (SHBG), thyroid-binding globulin (TBG), and other serum proteins and suppress follicle-stimulating hormone (FSH) from the anterior pituitary. Increases in the down-stream effects of ER binding reverses some of the symptoms of menopause, which are primarily caused by a loss of estrogenic activity.

GDUFA
DMF Review : Reviewed
Rev. Date : 2017-06-02
Pay. Date : 2017-04-04
DMF Number : 23948
Submission : 2010-07-02
Status : Active
Type : II

Registration Number : 303MF10163
Registrant's Address : Parc Industriel d'Incarville Parc de la Fringale CS 10606 27106 Val de Reuil Cedex France
Initial Date of Registration : 2021-11-10
Latest Date of Registration :

NDC Package Code : 24823-906
Start Marketing Date : 2011-04-07
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
Minakem delivers API, HPAPI, steroids & CDMO services for generics with FDA/GMP certification, regulatory know-how & proven success.
 Androst Biotech delivering high-quality hormone APIs and intermediates with trusted global compliance and competitive excellence.
Androst Biotech delivering high-quality hormone APIs and intermediates with trusted global compliance and competitive excellence.
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3072
Submission : 1977-11-09
Status : Inactive
Type : II

 Transo-Pharm GmbH works globally to supply Active Pharmaceutical Ingredients adhering to the highest quality & GMP standards.
Transo-Pharm GmbH works globally to supply Active Pharmaceutical Ingredients adhering to the highest quality & GMP standards.

GDUFA
DMF Review : Reviewed
Rev. Date : 2015-01-06
Pay. Date : 2014-12-22
DMF Number : 12268
Submission : 1996-12-16
Status : Active
Type : II

Certificate Number : CEP 2009-171 - Rev 02
Issue Date : 2025-04-25
Type : Chemical
Substance Number : 1614
Status : Valid

NDC Package Code : 60870-0276
Start Marketing Date : 1980-04-25
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Registrant Name : Synex Co., Ltd.
Registration Date : 2022-05-26
Registration Number : 20220526-181-I-638-07
Manufacturer Name : Aspen Oss BV@Aspen Oss BV
Manufacturer Address : Kloosterstraat 6, Oss, 5349 AB, The Netherlands@Veersemeer 4, Oss, 5347 JN, The Netherlands

| Available Reg Filing : ASMF, BR |
 SWATI - Transforming science into solutions with 60+ years of expertise, global accreditations, and pioneering biotech innovation.
SWATI - Transforming science into solutions with 60+ years of expertise, global accreditations, and pioneering biotech innovation.

NDC Package Code : 24823-905
Start Marketing Date : 2010-02-23
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2017-06-02
Pay. Date : 2017-04-04
DMF Number : 23948
Submission : 2010-07-02
Status : Active
Type : II
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3072
Submission : 1977-11-09
Status : Inactive
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2015-01-06
Pay. Date : 2014-12-22
DMF Number : 12268
Submission : 1996-12-16
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4267
Submission : 1981-09-03
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24553
Submission : 2011-01-18
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25854
Submission : 2012-03-30
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2025-10-14
Pay. Date : 2025-09-24
DMF Number : 39594
Submission : 2024-03-20
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
12
PharmaCompass offers a list of Estradiol Valerate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Estradiol Valerate manufacturer or Estradiol Valerate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Estradiol Valerate manufacturer or Estradiol Valerate supplier.
PharmaCompass also assists you with knowing the Estradiol Valerate API Price utilized in the formulation of products. Estradiol Valerate API Price is not always fixed or binding as the Estradiol Valerate Price is obtained through a variety of data sources. The Estradiol Valerate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Estradiol Valerate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Estradiol Valerate, including repackagers and relabelers. The FDA regulates Estradiol Valerate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Estradiol Valerate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Estradiol Valerate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Estradiol Valerate supplier is an individual or a company that provides Estradiol Valerate active pharmaceutical ingredient (API) or Estradiol Valerate finished formulations upon request. The Estradiol Valerate suppliers may include Estradiol Valerate API manufacturers, exporters, distributors and traders.
click here to find a list of Estradiol Valerate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Estradiol Valerate DMF (Drug Master File) is a document detailing the whole manufacturing process of Estradiol Valerate active pharmaceutical ingredient (API) in detail. Different forms of Estradiol Valerate DMFs exist exist since differing nations have different regulations, such as Estradiol Valerate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Estradiol Valerate DMF submitted to regulatory agencies in the US is known as a USDMF. Estradiol Valerate USDMF includes data on Estradiol Valerate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Estradiol Valerate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Estradiol Valerate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Estradiol Valerate Drug Master File in Japan (Estradiol Valerate JDMF) empowers Estradiol Valerate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Estradiol Valerate JDMF during the approval evaluation for pharmaceutical products. At the time of Estradiol Valerate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Estradiol Valerate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Estradiol Valerate Drug Master File in Korea (Estradiol Valerate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Estradiol Valerate. The MFDS reviews the Estradiol Valerate KDMF as part of the drug registration process and uses the information provided in the Estradiol Valerate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Estradiol Valerate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Estradiol Valerate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Estradiol Valerate suppliers with KDMF on PharmaCompass.
A Estradiol Valerate CEP of the European Pharmacopoeia monograph is often referred to as a Estradiol Valerate Certificate of Suitability (COS). The purpose of a Estradiol Valerate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Estradiol Valerate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Estradiol Valerate to their clients by showing that a Estradiol Valerate CEP has been issued for it. The manufacturer submits a Estradiol Valerate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Estradiol Valerate CEP holder for the record. Additionally, the data presented in the Estradiol Valerate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Estradiol Valerate DMF.
A Estradiol Valerate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Estradiol Valerate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Estradiol Valerate suppliers with CEP (COS) on PharmaCompass.
A Estradiol Valerate written confirmation (Estradiol Valerate WC) is an official document issued by a regulatory agency to a Estradiol Valerate manufacturer, verifying that the manufacturing facility of a Estradiol Valerate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Estradiol Valerate APIs or Estradiol Valerate finished pharmaceutical products to another nation, regulatory agencies frequently require a Estradiol Valerate WC (written confirmation) as part of the regulatory process.
click here to find a list of Estradiol Valerate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Estradiol Valerate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Estradiol Valerate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Estradiol Valerate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Estradiol Valerate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Estradiol Valerate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Estradiol Valerate suppliers with NDC on PharmaCompass.
Estradiol Valerate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Estradiol Valerate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Estradiol Valerate GMP manufacturer or Estradiol Valerate GMP API supplier for your needs.
A Estradiol Valerate CoA (Certificate of Analysis) is a formal document that attests to Estradiol Valerate's compliance with Estradiol Valerate specifications and serves as a tool for batch-level quality control.
Estradiol Valerate CoA mostly includes findings from lab analyses of a specific batch. For each Estradiol Valerate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Estradiol Valerate may be tested according to a variety of international standards, such as European Pharmacopoeia (Estradiol Valerate EP), Estradiol Valerate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Estradiol Valerate USP).

