
Synopsis
Synopsis
0
KDMF
0
VMF
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
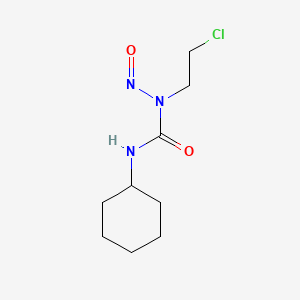

1. Belustine
2. Ccnu
3. Cecenu
4. Ceenu
5. Nsc 79037
6. Nsc-79037
7. Nsc79037
1. 13010-47-4
2. 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea
3. Ccnu
4. Belustine
5. Ceenu
6. Cecenu
7. Cinu
8. Chloroethylcyclohexylnitrosourea
9. Urea, N-(2-chloroethyl)-n'-cyclohexyl-n-nitroso-
10. Lomustina
11. Lomustinum
12. Lomustinum [inn-latin]
13. Lomustina [inn-spanish]
14. N-(2-chloroethyl)-n'-cyclohexyl-n-nitrosourea
15. 1-(2-chloroethyl)-3-cyclohexylnitrosourea
16. Nsc 79037
17. Nsc-79037
18. Gleostine
19. Cyclohexyl Chloroethyl Nitrosourea
20. Sri 2200
21. Icig 1109
22. Nci-c04740
23. (chloro-2-ethyl)-1-cyclohexyl-3-nitrosourea
24. Rb 1509
25. (cloro-2-etil)-1-cicloesil-3-nitrosourea
26. Nsc79037
27. Lomustine (ceenu)
28. 1-nitrosourea, 1-(2-chloroethyl)-3-cyclohexyl-
29. Chebi:6520
30. Urea, 1-(2-chloroethyl)-3-cyclohexyl-1-nitroso-
31. Ccn-u
32. 7brf0z81kg
33. Ccnu; Nsc 79037
34. 1-(2-chloroethyl)-3-cyclohexyl-1-nitroso-urea
35. Ncgc00167466-01
36. Dsstox_cid_3222
37. 3-(2-chloroethyl)-1-cyclohexyl-3-nitrosourea
38. Dsstox_rid_76930
39. Dsstox_gsid_23222
40. Ccris 860
41. Cas-13010-47-4
42. Ccnu [chloroethyl Nitrosoureas]
43. Hsdb 6519
44. Sr-05000001497
45. Einecs 235-859-2
46. Unii-7brf0z81kg
47. Brn 2125058
48. (cloro-2-etil)-1-cicloesil-3-nitrosourea [italian]
49. Lomustine [usan:inn:ban]
50. Ai3-52779
51. Gleostine (tn)
52. Lomustine- Bio-x
53. Lomustine, >=98%
54. Lomustine (usp/inn)
55. Lomustine [inn]
56. Lomustine [mi]
57. Lomustine [hsdb]
58. Lomustine [usan]
59. Lomustine [vandf]
60. 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea [chloroethyl Nitrosoureas]
61. Chembl514
62. Lomustine [mart.]
63. Ncimech_000220
64. Lomustine [usp-rs]
65. Lomustine [who-dd]
66. Schembl3995
67. Wln: L6tj Amvnno&2g
68. Gtpl7214
69. Lomustine [orange Book]
70. Dtxsid2023222
71. Lomustine [ep Monograph]
72. Lomustine [usp Monograph]
73. Hms2090a17
74. Hms3655i16
75. Pharmakon1600-01502301
76. Bcp06551
77. Zinc3831006
78. Tox21_112470
79. Bdbm50247919
80. Mfcd00012392
81. Nsc759635
82. S1840
83. Akos005766022
84. Tox21_112470_1
85. Ac-8062
86. Ccg-213022
87. Cs-1461
88. Db01206
89. Ds-1269
90. Nsc-759635
91. Ncgc00167466-02
92. Ncgc00167466-03
93. Bl164634
94. Hy-13669
95. Nci60_041743
96. Db-017097
97. Am20070540
98. Ft-0627972
99. L0251
100. Sw220040-1
101. C07079
102. D00363
103. Ab00173884-02
104. Ab00173884-03
105. Ab00173884-04
106. Ab00173884_05
107. Ab00173884_06
108. 010l474
109. A806019
110. Q415378
111. Sr-05000001497-1
112. Sr-05000001497-3
113. Urea, 1-(2-chloroethyl)-3-cyclohexyl)-1-nitroso-
114. W-108355
115. Lomustine, European Pharmacopoeia (ep) Reference Standard
116. 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea [iarc]
117. Lomustine, United States Pharmacopeia (usp) Reference Standard
118. 1-(2-chloroethyl)-1-[(cyclohexylamino)carbonyl]-2-oxohydrazine #
119. 2-chloro-n-(cyclohexyl-c-hydroxycarbonimidoyl)-n-nitrosoethan-1-amine
120. 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea1-(2-chloroethyl)-3-cyclohexyl-1-nitroso-urea13010-47-4nci60_0417431-(2-chloroethyl)-3-cyclohexylnitrosourea1-nitrosourea, 1-(2-chloroethyl)-3-cyclohexyl-be
| Molecular Weight | 233.69 g/mol |
|---|---|
| Molecular Formula | C9H16ClN3O2 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 233.0931045 g/mol |
| Monoisotopic Mass | 233.0931045 g/mol |
| Topological Polar Surface Area | 61.8 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 219 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents, Alkylating
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
CeeNU has been shown to be useful as a single agent in addition to other treatment modalities, or in established combination therapy with other approved chemotherapeutic agents in the following: Brain tumors-both primary and metastatic, in patients who have already received appropriate surgical and/or radiotherapeutic procedures. Hodgkin's Disease-secondary therapy in combination with other approved drugs in patients who relapse while being treated with primary therapy, or who fail to respond to primary therapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for CeeNU (lomustine) (June 2009). Available from, as of November 4, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10860
Antineoplastic agent. The compound has had limited use since the early 1970s in the treatment of Hodgkin's disease and various solid tumors. These include primary and metastatic brain tumors, colorectal tumors, and certain pulmonary malignancies. It is usually used in conjunction with other antineoplastic drugs.
DHHS/National Toxicology Program; Eleventh Report on Carcinogens: Lomustine (1-(2-Chloroethyl)-3-Cyclohexyl-1-Nitrosourea) (13010-47-4) (January 2005). Available from, as of September 29, 2009: https://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s035ccnu.pdf
Although lomustine is labeled for use in combination with other agents as secondary therapy for the treatment of refractory or relapsed Hodgkin's disease, combination regimens containing other agents currently are preferred for this cancer. /Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1153
For more Therapeutic Uses (Complete) data for LOMUSTINE (8 total), please visit the HSDB record page.
/BOXED WARNING/ WARNINGS: CeeNU (lomustine) should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Bone marrow suppression, notably thrombocytopenia and leukopenia, which may contribute to bleeding and overwhelming infections in an already compromised patient, is the most common and severe of the toxic effects of CeeNU. Since the major toxicity is delayed bone marrow suppression, blood counts should be monitored weekly for at least 6 weeks after a dose. At the recommended dosage, courses of CeeNU should not be given more frequently than every 6 weeks. The bone marrow toxicity of CeeNU is cumulative and therefore dosage adjustment must be considered on the basis of nadir blood counts from prior dose
US Natl Inst Health; DailyMed. Current Medication Information for CeeNU (lomustine) (Updated: November 2012). Available from, as of April 24, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=17893de9-7d54-448c-9fca-d10642046d14
Because some lomustine metabolites are present in milk, women receiving the drug probably should not nurse their infants.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1154
Delayed onset of pulmonary fibrosis occurring up to 17 years after treatment has been reported in patients receiving related nitrosoureas combined with cranial radiation therapy for intracranial tumors during childhood and adolescence (age 1-16 years). Late onset of reduction in pulmonary function was observed in all long-term survivors. Nitrosourea-induced pulmonary fibrosis may be slowly progressive and can cause death.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1153
Nausea and vomiting occur in 45-100% of patients within 45 min to 6 hr after ingestion of an oral dose of lomustine. Although these symptoms are not severe and usually abate within 24 hr, they may persist up to 36 hr and are often followed by 2-3 days of anorexia. Stomatitis has occurred infrequently.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1153
For more Drug Warnings (Complete) data for LOMUSTINE (25 total), please visit the HSDB record page.
For the treatment of primary and metastatic brain tumors as a component of combination chemotherapy in addition to appropriate surgical and/or radiotherapeutic procedures. Also used in combination with other agents as secondary therapy for the treatment of refractory or relapsed Hodgkin's disease.
Lomustine is an alkylating agent of the nitrosourea type. Lomustine and its metabolites interferes with the function of DNA and RNA. It is cell cycle–phase nonspecific. Cancers form when some cells within the body multiply uncontrollably and abnormally. These cells then spread and destroy nearby tissues. Lomustine acts by slowing this process down. It kills cancer cells by damaging the DNA (the genetic material inside the cells) and stops them from dividing.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AD - Nitrosoureas
L01AD02 - Lomustine
Absorption
Well and rapidly absorbed from the gastrointestinal tract.
Route of Elimination
Following oral administration of radioactive CeeNU at doses ranging from 30 mg/m2 to 100 mg/m2, about half of the radioactivity given was excreted in the urine in the form of degradation products within 24 hours.
Lomustine is excreted primarily in the urine as metabolites. Following oral administration of (14)C-labeled lomustine, about 50% of the radioactivity is excreted within 12 hr and about 75% within 4 days.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1154
Lomustine is reported to be widely distributed. Lomustine and/or its metabolites cross the blood-brain barrier and are rapidly transported into cells due to their high lipid solubility. Although intact lomustine is not detectable in the CSF, active metabolites of the drug appear in substantial concentrations within 30 minutes after oral administration of lomustine. CSF concentrations of metabolites have been reported to be 15-50% or greater than concurrent plasma concentrations. Lomustine metabolites are present in milk, but in concentrations than those in maternal plasma.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1154
Lomustine is rapidly absorbed from the GI tract; the drug is also absorbed following topical application. Peak plasma concentrations of metabolites occur within 1-6 hours following administration of an oral dose of lomustine.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1154
Following its ip or iv injection or oral administration, (14)C-labelled CCNU was rapidly distributed to many tissues in mice, rats, rabbits and dogs.. About 80% of label was excreted in the urine of mice 24 hours after a single parenteral or oral dose of 50 mg/kg bw.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V26 142 (1981)
Hepatic. Rapid and complete, with active metabolites.
CCNU undergoes spontaneous decomposition under physiological conditions to release both alkylating and carbamoylating entities. It disappears from plasma within 5 minutes following its oral administration, but the antitumor effect of its metabolites may persist for up to 15 minutes. ... In addition to chemical decomposition, CCNU may be converted by microsomal metabolism to 6 isomeric hydroxylated derivatives, some of which may differ in their biological properties from CCNU.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V26 142 (1981)
Virtually all of a dose of lomustine is metabolized within 1 hour after oral administration. The half-life of lomustine metabolites is biphasic; although the initial plasma half-life is 6 hours, the second phase plasma half-life is 1-2 days, and 15-20% of the metabolites remain in the body 5 days after administration of lomustine. Prolongation of plasma concentrations is thought to reflect a combination of protein binding and enterohepatic circulation of metabolites.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1154
Approximately 94 minutes, however the metabolites have a serum half-life of 16 to 48 hours.
The half-life of lomustine metabolites is biphasic; although the initial plasma half-life is 6 hours, the second phase plasma half-life is 1-2 days, and 15-20% of the metabolites remain in the body 5 days after administration of lomustine.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1154
Lomustine is a highly lipophilic nitrosourea compound which undergoes hydrolysis in vivo to form reactive metabolites. These metabolites cause alkylation and cross-linking of DNA (at the O6 position of guanine-containing bases) and RNA, thus inducing cytotoxicity. Other biologic effects include inhibition of DNA synthesis and some cell cycle phase specificity. Nitrosureas generally lack cross-resistance with other alkylating agents. As lomustine is a nitrosurea, it may also inhibit several key processes such as carbamoylation and modification of cellular proteins.
Although lomustine is believed to act by alkylation, the mechanism of action has not been completely elucidated, and other effects as carbamoylation and modification of cellular proteins may be involved. The overall result is thought to be the inhibition of both DNA and RNA synthesis.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 1154

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
74
PharmaCompass offers a list of Lomustine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lomustine manufacturer or Lomustine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lomustine manufacturer or Lomustine supplier.
PharmaCompass also assists you with knowing the Lomustine API Price utilized in the formulation of products. Lomustine API Price is not always fixed or binding as the Lomustine Price is obtained through a variety of data sources. The Lomustine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Lomustine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Lomustine, including repackagers and relabelers. The FDA regulates Lomustine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Lomustine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Lomustine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Lomustine supplier is an individual or a company that provides Lomustine active pharmaceutical ingredient (API) or Lomustine finished formulations upon request. The Lomustine suppliers may include Lomustine API manufacturers, exporters, distributors and traders.
click here to find a list of Lomustine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Lomustine DMF (Drug Master File) is a document detailing the whole manufacturing process of Lomustine active pharmaceutical ingredient (API) in detail. Different forms of Lomustine DMFs exist exist since differing nations have different regulations, such as Lomustine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Lomustine DMF submitted to regulatory agencies in the US is known as a USDMF. Lomustine USDMF includes data on Lomustine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Lomustine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Lomustine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Lomustine Drug Master File in Japan (Lomustine JDMF) empowers Lomustine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Lomustine JDMF during the approval evaluation for pharmaceutical products. At the time of Lomustine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Lomustine suppliers with JDMF on PharmaCompass.
A Lomustine CEP of the European Pharmacopoeia monograph is often referred to as a Lomustine Certificate of Suitability (COS). The purpose of a Lomustine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Lomustine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Lomustine to their clients by showing that a Lomustine CEP has been issued for it. The manufacturer submits a Lomustine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Lomustine CEP holder for the record. Additionally, the data presented in the Lomustine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Lomustine DMF.
A Lomustine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Lomustine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Lomustine suppliers with CEP (COS) on PharmaCompass.
A Lomustine written confirmation (Lomustine WC) is an official document issued by a regulatory agency to a Lomustine manufacturer, verifying that the manufacturing facility of a Lomustine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Lomustine APIs or Lomustine finished pharmaceutical products to another nation, regulatory agencies frequently require a Lomustine WC (written confirmation) as part of the regulatory process.
click here to find a list of Lomustine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Lomustine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Lomustine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Lomustine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Lomustine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Lomustine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Lomustine suppliers with NDC on PharmaCompass.
Lomustine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Lomustine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Lomustine GMP manufacturer or Lomustine GMP API supplier for your needs.
A Lomustine CoA (Certificate of Analysis) is a formal document that attests to Lomustine's compliance with Lomustine specifications and serves as a tool for batch-level quality control.
Lomustine CoA mostly includes findings from lab analyses of a specific batch. For each Lomustine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Lomustine may be tested according to a variety of international standards, such as European Pharmacopoeia (Lomustine EP), Lomustine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Lomustine USP).

