
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
Weekly News Recap #Phispers
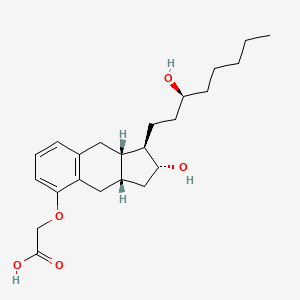

1. ((1r,2r,3as,9as)-2-hydroxy-1-((3s)-3-hydroxyoctyl)-2,3,3a,4,9,9a-hexahydro-1h-cylopent(b)naphthalen-5-yl)oxy)acetate
2. Orenitram
3. Remodulin
4. Trepostinil Sodium
5. Treprostinil Diethanolamine
6. Treprostinil Diolamin
7. Treprostinil Diolamine
8. Treprostinil Sodium
9. Ut-15
10. Ut-15c
1. 81846-19-7
2. Remodulin
3. Uniprost
4. Rumodolin
5. Orenitram
6. Tyvaso
7. Lrx-15
8. Ut-15
9. 15au81
10. Treprostinil Free Acid
11. Rum6k67esg
12. 2-(((1r,2r,3as,9as)-2-hydroxy-1-((s)-3-hydroxyoctyl)-2,3,3a,4,9,9a-hexahydro-1h-cyclopenta[b]naphthalen-5-yl)oxy)acetic Acid
13. Chebi:50861
14. 81846-19-7 (free Acid)
15. ((1r,2r,3as,9as)-2-hydroxy-1-((3s)-3-hydroxyoctyl)-2,3,3a,4,9,9a-hexahydro-1h-cylopent(b)naphthalen-5-yl)oxy)acetate
16. 2-[[(1r,2r,3as,9as)-2-hydroxy-1-[(3s)-3-hydroxyoctyl]-2,3,3a,4,9,9a-hexahydro-1h-cyclopenta[g]naphthalen-5-yl]oxy]acetic Acid
17. 289480-64-4
18. 15-au-81
19. L-606
20. Treprostinilo
21. Treprostinilum
22. Lrx 15
23. Treprostinil [usan:inn]
24. Remodulin (tn)
25. Unii-rum6k67esg
26. U 62840
27. Tresprostinil
28. Trevyent
29. U-62,840
30. Treprostinil [mi]
31. Treprostinil [inn]
32. Treprostinil [jan]
33. Treprostinil [usan]
34. Treprostinil [vandf]
35. Treprostinil Pound>>ut-15
36. Treprostinil [mart.]
37. Treprostinil [who-dd]
38. 15au
39. Gtpl5820
40. Schembl4349618
41. Treprostinil (jan/usan/inn)
42. Chembl1237119
43. Lrx -15
44. Dtxsid901021654
45. Hms3648g07
46. Treprostinil [orange Book]
47. Amy22230
48. Bcp10253
49. Ex-a1414
50. Zinc3800475
51. Mfcd00888847
52. Akos027470173
53. Cs-7872
54. Db00374
55. Ncgc00343944-03
56. ({(1r,2r,3as,9as)-2-hydroxy-1-[(3s)-3-hydroxyoctyl]-2,3,3a,4,9,9a-hexahydro-1h-cyclopenta[b]naphthalen-5-yl}oxy)acetic Acid
57. 2-[[(1r,2r,3as,9as)-2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-[(3s)-3-hydroxyoctyl]-1h-benz[f]inden-5-yl]oxy]acetic Acid
58. Ac-30207
59. As-56364
60. L606
61. Hy-100441
62. D06213
63. Sr-01000946210
64. Q3495231
65. Sr-01000946210-1
66. Brd-k19706299-001-01-4
67. [[(1r,2r,3as,9as)-2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-[(s)-3-hydroxyoctyl]-1h-benzo[f]indene-5-yl]oxy]acetic Acid
68. [[(1r,2r,3as,9as)-2,3,4,9,9a-hexahydro-2-hydroxy-1-[(3s)-3-hydroxyoctyl]-1h-benz[f]inden-5-yl]oxy]acetic Acid
69. 2-((1r,2r,3as,9as)-2-hydroxy-1-((s)-3-hydroxyoctyl)-2,3,3a,4,9,9a-hexahydro-1h-cyclopenta[b]naphthalen-5-yloxy)acetic Acid
70. 2-[[(1r,2r,3as,9as)-2,3,4,9,9a-hexahydro-2-hydroxy-1-[(3s)-3-hydroxyoctyl]-1h-benz[f]inden-5-yl]oxy]-acetic Acid
71. 2-[[(2r,3r,3as,9as)-2-hydroxy-3-[(3s)-3-hydroxyoctyl]-2,3,3a,4,9,9a-hexahydro-1h-cyclopenta[g]naphthalen-8-yl]oxy]acetic Acid
72. 2-{[(1r,2r,3as,9as)-2-hydroxy-1-[(3s)-3-hydroxyoctyl]-1h,2h,3h,3ah,4h,9h,9ah-cyclopenta[b]naphthalen-5-yl]oxy}acetic Acid
73. Acetic Acid, (((1r,2r,3as,9as)-2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-((3s)-3-hydroxyoctyl)-1h-benz(f)inden-5-yl)oxy)-
74. Acetic Acid, ((2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-(3-hydroxyoctyl)-1h-benz(f)inden-5-yl)oxy)-, (1r-(1alpha(s*),2beta,3aalpha,9aalpha))-
75. Acetic Acid,(((1r,2r,3as,9as)-2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-((3s)-3-hydroxyoctyl)-1h-benz(f)inden-5-yl)oxy)-
| Molecular Weight | 390.5 g/mol |
|---|---|
| Molecular Formula | C23H34O5 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 390.24062418 g/mol |
| Monoisotopic Mass | 390.24062418 g/mol |
| Topological Polar Surface Area | 87 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 495 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Remodulin |
| PubMed Health | Treprostinil |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Platelet Aggregation Inhibitor, Vasodilator |
| Drug Label | Remodulin (treprostinil) Injection is a sterile solution of treprostinil formulated for subcutaneous or intravenous administration. Remodulin is supplied in 20 mL multidose vials in four strengths, containing 20 mg, 50 mg, 100 mg, or 200 mg (1 mg/mL,... |
| Active Ingredient | Treprostinil |
| Dosage Form | Injectable |
| Route | Iv (infusion), subcutaneous |
| Strength | 1mg/ml; 2.5mg/ml; 10mg/ml; 5mg/ml |
| Market Status | Prescription |
| Company | United Therap |
| 2 of 4 | |
|---|---|
| Drug Name | Tyvaso |
| PubMed Health | Treprostinil (By breathing) |
| Drug Classes | Vasodilator |
| Drug Label | Tyvaso is a sterile formulation of treprostinil intended for administration by oral inhalation using the Tyvaso Inhalation System. Tyvaso is supplied in 2.9 mL low density polyethylene (LDPE) ampules, containing 1.74 mg treprostinil (0.6 mg/mL). Each... |
| Active Ingredient | Treprostinil |
| Dosage Form | Solution |
| Route | Inhalation |
| Strength | eq 0.6mg base/ml |
| Market Status | Prescription |
| Company | United Therap |
| 3 of 4 | |
|---|---|
| Drug Name | Remodulin |
| PubMed Health | Treprostinil |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Platelet Aggregation Inhibitor, Vasodilator |
| Drug Label | Remodulin (treprostinil) Injection is a sterile solution of treprostinil formulated for subcutaneous or intravenous administration. Remodulin is supplied in 20 mL multidose vials in four strengths, containing 20 mg, 50 mg, 100 mg, or 200 mg (1 mg/mL,... |
| Active Ingredient | Treprostinil |
| Dosage Form | Injectable |
| Route | Iv (infusion), subcutaneous |
| Strength | 1mg/ml; 2.5mg/ml; 10mg/ml; 5mg/ml |
| Market Status | Prescription |
| Company | United Therap |
| 4 of 4 | |
|---|---|
| Drug Name | Tyvaso |
| PubMed Health | Treprostinil (By breathing) |
| Drug Classes | Vasodilator |
| Drug Label | Tyvaso is a sterile formulation of treprostinil intended for administration by oral inhalation using the Tyvaso Inhalation System. Tyvaso is supplied in 2.9 mL low density polyethylene (LDPE) ampules, containing 1.74 mg treprostinil (0.6 mg/mL). Each... |
| Active Ingredient | Treprostinil |
| Dosage Form | Solution |
| Route | Inhalation |
| Strength | eq 0.6mg base/ml |
| Market Status | Prescription |
| Company | United Therap |
For use as a continuous subcutaneous infusion or intravenous infusion (for those not able to tolerate a subcutaneous infusion) for the treatment of pulmonary arterial hypertension in patients with NYHA Class II-IV symptoms to diminish symptoms associated with exercise.
FDA Label
Treatment of adult patients with WHO Functional Class (FC) III or IV and:
- inoperable chronic thromboembolic pulmonary hypertension (CTEPH), or
- persistent or recurrent CTEPH after surgical treatment
to improve exercise capacity.
Treatment of pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is a disease in which blood pressure is abnormally high in the arteries between the heart and lungs. PAH is characterized by symptoms of shortness of breath during physical exertion. The condition can ultimately lead to heart failure. Treprostinil is a potent oral antiplatelet agent. The major pharmacologic actions of treprostinil are direct vasodilation of pulmonary and systemic arterial vascular beds and inhibition of platelet aggregation. In animals, the vasodilatory effects reduce right and left ventricular afterload and increase cardiac output and stroke volume. Other studies have shown that treprostinil causes a dose-related negative inotropic and lusitropic effect. No major effects on cardiac conduction have been observed.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
B01AC21
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC21 - Treprostinil
Absorption
Relatively rapid and complete after subcutaneous infusion, with an absolute bioavailability approximately 100%. In patients with mild (n=4) or moderate (n=5) hepatic insufficiency and portopulmonary hypertension following a subcutaneous dose of 10 ng per kg of body weight per min for 150 mins the AUC 0-∞ was increased 3-fold and 5-fold respectively.
Volume of Distribution
14 L/70 kg
Substantially metabolized by the liver, but the precise enzymes responsible are unknown. Five metabolites have been described (HU1 through HU5) however, the biological activity and metabolic fate of these are unknown. The chemical structure of HU1 is unknown. The metabolite HU5 is the glucuronide conjugate of treprostinil. The other metabolites are formed by oxidation of the 3-hydroxyoctyl side chain (HU2) and subsequent additional oxidation (HU3) or dehydration (HU4). Study results of in vitro human hepatic cytochrome P450 demonstrates that treprostinil does not inhibit CYP-1A2, 2C9, 2C19, 2D6, 2E1, or 3A. There have been no studies that evaluate the potential of treprostinil to induce these enzymes.
Terminal elimination half-life is approximately 2 to 4 hours. Plasma half-life is 34 and 85 minutes for intravenous and subcutaneous infusion of the drug, respectively.
The major pharmacological actions of treprostinil are direct vasodilation of pulmonary and systemic arterial vascular beds and inhibition of platelet aggregation. In addition to treprostinil's direct vasodilatory effects, it also inhibits inflammatory cytokine. As a synthetic analogue of prostacyclin, it binds to the prostacyclin receptor, which subsequently induces the aforementioned downstream effects.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
44
PharmaCompass offers a list of Treprostinil Sodium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Treprostinil Sodium manufacturer or Treprostinil Sodium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Treprostinil Sodium manufacturer or Treprostinil Sodium supplier.
PharmaCompass also assists you with knowing the Treprostinil Sodium API Price utilized in the formulation of products. Treprostinil Sodium API Price is not always fixed or binding as the Treprostinil Sodium Price is obtained through a variety of data sources. The Treprostinil Sodium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Treprostinil Sodium manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Treprostinil Sodium, including repackagers and relabelers. The FDA regulates Treprostinil Sodium manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Treprostinil Sodium API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Treprostinil Sodium manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Treprostinil Sodium supplier is an individual or a company that provides Treprostinil Sodium active pharmaceutical ingredient (API) or Treprostinil Sodium finished formulations upon request. The Treprostinil Sodium suppliers may include Treprostinil Sodium API manufacturers, exporters, distributors and traders.
click here to find a list of Treprostinil Sodium suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Treprostinil Sodium DMF (Drug Master File) is a document detailing the whole manufacturing process of Treprostinil Sodium active pharmaceutical ingredient (API) in detail. Different forms of Treprostinil Sodium DMFs exist exist since differing nations have different regulations, such as Treprostinil Sodium USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Treprostinil Sodium DMF submitted to regulatory agencies in the US is known as a USDMF. Treprostinil Sodium USDMF includes data on Treprostinil Sodium's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Treprostinil Sodium USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Treprostinil Sodium suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Treprostinil Sodium Drug Master File in Korea (Treprostinil Sodium KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Treprostinil Sodium. The MFDS reviews the Treprostinil Sodium KDMF as part of the drug registration process and uses the information provided in the Treprostinil Sodium KDMF to evaluate the safety and efficacy of the drug.
After submitting a Treprostinil Sodium KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Treprostinil Sodium API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Treprostinil Sodium suppliers with KDMF on PharmaCompass.
A Treprostinil Sodium written confirmation (Treprostinil Sodium WC) is an official document issued by a regulatory agency to a Treprostinil Sodium manufacturer, verifying that the manufacturing facility of a Treprostinil Sodium active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Treprostinil Sodium APIs or Treprostinil Sodium finished pharmaceutical products to another nation, regulatory agencies frequently require a Treprostinil Sodium WC (written confirmation) as part of the regulatory process.
click here to find a list of Treprostinil Sodium suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Treprostinil Sodium as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Treprostinil Sodium API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Treprostinil Sodium as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Treprostinil Sodium and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Treprostinil Sodium NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Treprostinil Sodium suppliers with NDC on PharmaCompass.
Treprostinil Sodium Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Treprostinil Sodium GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Treprostinil Sodium GMP manufacturer or Treprostinil Sodium GMP API supplier for your needs.
A Treprostinil Sodium CoA (Certificate of Analysis) is a formal document that attests to Treprostinil Sodium's compliance with Treprostinil Sodium specifications and serves as a tool for batch-level quality control.
Treprostinil Sodium CoA mostly includes findings from lab analyses of a specific batch. For each Treprostinil Sodium CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Treprostinil Sodium may be tested according to a variety of international standards, such as European Pharmacopoeia (Treprostinil Sodium EP), Treprostinil Sodium JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Treprostinil Sodium USP).

