16 Dec 2025
// PRESS RELEASE
10 Dec 2025
// PRESS RELEASE
23 Oct 2025
// PRESS RELEASE
 KEY PRODUCTS
KEY PRODUCTS KEY SERVICES
KEY SERVICES
EUROAPI, the leading small molecules API player, provides both API sales & CDMO services.
About
Village de la ChimieVillage de la Chimie
Industry Trade Show
Attending
18-19 February, 2026
World ADC LondonWorld ADC London
Industry Trade Show
Attending
26 February, 2026
Industry Trade Show
Hilton Midtown
23-26 March, 2026
CONTACT DETAILS





Events
Webinars & Exhibitions
Village de la ChimieVillage de la Chimie
Industry Trade Show
Attending
18-19 February, 2026
World ADC LondonWorld ADC London
Industry Trade Show
Attending
26 February, 2026
Industry Trade Show
Hilton Midtown
23-26 March, 2026
CORPORATE CONTENT #SupplierSpotlight
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-veranova-chemexpress-invest-in-adc-facilities-cohance-to-set-up-oligonucleotide-facility-in-india
https://www.pharmacompass.com/radio-compass-blog/global-api-micronization-market-set-to-surge-49-by-2030-led-by-specialized-industry-pioneers
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-veranova-carbogen-lead-adc-investments-axplora-polfa-tarchomin-famar-expand-european-footprint
https://www.pharmacompass.com/radio-compass-blog/bms-j-j-bayer-lead-25-000-pharma-layoffs-in-2024-amylyx-fibrogen-kronos-bio-hit-by-trial-failures-cash-crunch
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-bora-polpharma-make-acquisitions-evonik-euroapi-porton-announce-technological-expansions
https://www.pharmacompass.com/radio-compass-blog/chinese-fda-registered-generic-facilities-gain-steam-india-maintains-lead-with-396-facilities
https://www.pharmacompass.com/radio-compass-blog/us-europe-turn-to-advanced-manufacturing-stockpiling-to-strengthen-drug-supply-chains
https://www.pharmacompass.com/radio-compass-blog/bms-bayer-takeda-pfizer-downsize-to-combat-cost-pressures-meet-restructuring-plans
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-novo-s-parent-buys-catalent-for-us-16-5-bn-fujifilm-merck-kgaa-axplora-lonza-expand-capabilities
https://www.pharmacompass.com/radio-compass-blog/fda-approves-four-oligonucleotide-therapies-in-2023-novartis-gsk-novo-bet-big

16 Dec 2025
// PRESS RELEASE
https://www.euroapi.com/sites/default/files/2025-12/euroapi-press-release-december-16-2025.pdf

10 Dec 2025
// PRESS RELEASE
https://www.euroapi.com/en/tristan-imbert-co-opted-as-independent-director

23 Oct 2025
// PRESS RELEASE
https://www.euroapi.com/en/with-med4cure-euroapi-accelerates-pharmaceutical-innovation-at-the-service-of-health-sovereignty

23 Oct 2025
// BUSINESSWIRE
https://www.businesswire.com/news/home/20251023325690/en/Abolis-Biotechnologies-and-EUROAPI-Enter-Into-Partnership-to-Accelerate-Pharmaceutical-Innovation-and-Restore-European-Sovereignty

14 Oct 2025
// PR NEWSWIRE
https://www.prnewswire.com/news-releases/codis-launches-as-global-cdmo-for-commercial-spray-drying-and-amorphous-solid-dispersions-302580144.html

30 Sep 2025
// PRESS RELEASE
Registration Number : 218MF10374
Registrant's Address : 82 Avenue Raspail 94250 Gentilly France
Initial Date of Registration : 2006-03-20
Latest Date of Registration : 2013-10-09
Registration Number : 220MF10064
Registrant's Address : To (´) utca 1-5. , 1045 Budapest, Hungary
Initial Date of Registration : 2008-02-25
Latest Date of Registration : 2025-11-05
Registration Number : 218MF10339
Registrant's Address : To(´) utca 1-5. , 1045 Budapest, Hungary
Initial Date of Registration : 2006-05-22
Latest Date of Registration : 2010-01-07
Registration Number : 303MF10151
Registrant's Address : To(´) utca 1-5. , 1045 Budapest, Hungary
Initial Date of Registration : 2021-09-17
Latest Date of Registration : 2021-09-17
Registration Number : 218MF10340
Registrant's Address : To(´) utca 1-5. , 1045 Budapest, Hungary
Initial Date of Registration : 2006-03-09
Latest Date of Registration : 2012-07-09
Registration Number : 229MF10098
Registrant's Address : To(´) utca 1-5. , 1045 Budapest, Hungary
Initial Date of Registration : 2017-05-24
Latest Date of Registration : 2017-05-24
Registration Number : 218MF10337
Registrant's Address : Brueningstrasse 50, 65926 Frankfurt am Main, Germany
Initial Date of Registration : 2006-03-09
Latest Date of Registration : 2010-01-07
Registration Number : 226MF10008
Registrant's Address : 82 Avenue Raspail 94250 Gentilly (FRANCE)
Initial Date of Registration : 2014-01-08
Latest Date of Registration : 2015-04-09
Registration Number : 222MF10121
Registrant's Address : 82 Avenue Raspail 94250 Gentilly France
Initial Date of Registration : 2010-04-02
Latest Date of Registration : 2020-02-06
Registration Number : 222MF10011
Registrant's Address : 15 rue Traversie(')re 75012 Paris France
Initial Date of Registration : 2010-01-07
Latest Date of Registration : 2024-02-07
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Services
API Manufacturing
API & Drug Product Development
Excipients
Excipients by Ingredients
Excipients By applications
Inspections and registrations
Country : France
City/Region : Saint Aubin Les Elbeuf
Audit Date : 2025-05-20
Audit Type : On-Site
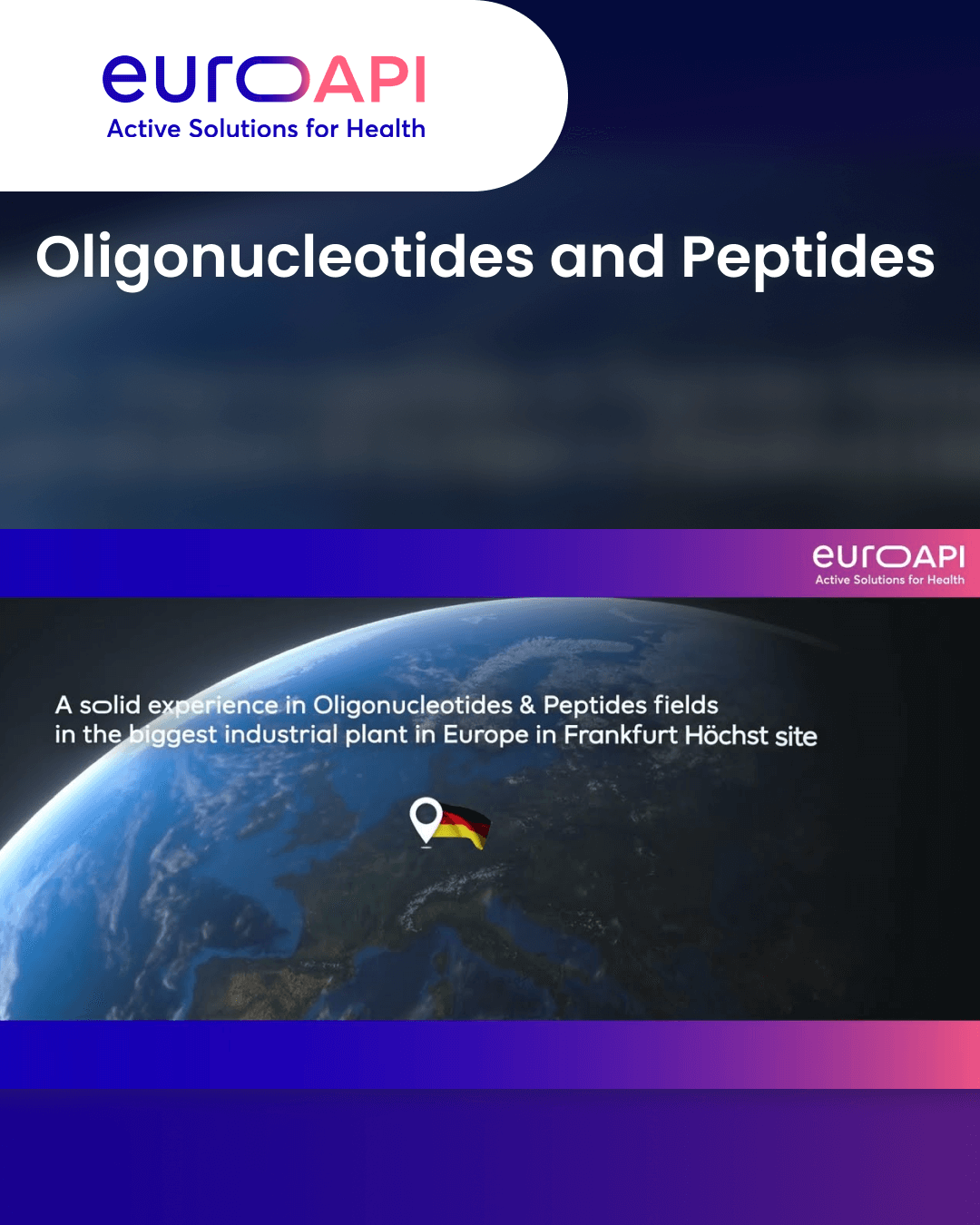
Country : Germany
City/Region : Frankfurt am Main
Audit Date : 2024-02-20
Audit Type : On-Site
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
EUROAPI is a supplier offers 140 products (APIs, Excipients or Intermediates).
Find a price of Alprostadil bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Clobazam bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Dexamethasone bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Dexamethasone Sodium Phosphate bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Hydrocortisone bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Hydroxocobalamin Acetate bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Ketoprofen bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Latanoprost bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Methylprednisolone Hemisuccinate bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Naloxone Hydrochloride bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Prednisolone bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Prednisolone Acetate bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Selegiline Hydrochloride bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Spironolactone bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Vitamin B12 bulk with DMF, CEP, JDMF offered by EUROAPI
Find a price of Apomorphine Hydrochloride bulk with DMF, CEP offered by EUROAPI
Find a price of Bimatoprost bulk with DMF, JDMF offered by EUROAPI
Find a price of Dexamethasone bulk with DMF, CEP offered by EUROAPI
Find a price of Dinoprostone bulk with DMF, JDMF offered by EUROAPI
Find a price of Fexofenadine Hydrochloride bulk with CEP, JDMF offered by EUROAPI
Find a price of Fluorometholone bulk with DMF, JDMF offered by EUROAPI
Find a price of Fluticasone Propionate bulk with CEP, JDMF offered by EUROAPI
Find a price of Glimepiride bulk with DMF, CEP offered by EUROAPI
Find a price of Hydrocortisone Acetate bulk with CEP, JDMF offered by EUROAPI
Find a price of Hydroxocobalamin bulk with DMF, JDMF offered by EUROAPI
Find a price of Irbesartan bulk with DMF, CEP offered by EUROAPI
Find a price of Methylprednisolone bulk with DMF, CEP offered by EUROAPI
Find a price of Naltrexone Hydrochloride bulk with DMF, CEP offered by EUROAPI
Find a price of Prednisone bulk with DMF, CEP offered by EUROAPI
Find a price of Ramipril bulk with DMF, CEP offered by EUROAPI
Find a price of Rifampicin bulk with DMF, JDMF offered by EUROAPI
Find a price of Rifaximin bulk with DMF, JDMF offered by EUROAPI
Find a price of Sevelamer Hydrochloride bulk with DMF, JDMF offered by EUROAPI
Find a price of Sodium Cromoglicate bulk with DMF, CEP offered by EUROAPI
Find a price of Travoprost bulk with DMF, JDMF offered by EUROAPI
Find a price of Alprostadil Alfadex bulk with JDMF offered by EUROAPI
Find a price of Beraprost Sodium bulk with JDMF offered by EUROAPI
Find a price of Bimatoprost bulk with DMF offered by EUROAPI
Find a price of Carboprost Tromethamine bulk with DMF offered by EUROAPI
Find a price of Clobazam bulk with CEP offered by EUROAPI
Find a price of Clopidogrel bulk with JDMF offered by EUROAPI
Find a price of Codeine bulk with CEP offered by EUROAPI
Find a price of Codeine Phosphate bulk with CEP offered by EUROAPI
Find a price of Cortisone bulk with JDMF offered by EUROAPI
Find a price of Desoximetasone bulk with DMF offered by EUROAPI
Find a price of Dexamethasone Sodium Phosphate bulk with JDMF offered by EUROAPI
Find a price of Dinoprost bulk with JDMF offered by EUROAPI
Find a price of Dinoprost Tromethamine bulk with CEP offered by EUROAPI
Find a price of Epoprostenol Sodium bulk with DMF offered by EUROAPI
Find a price of Ethylmorphine Hydrochloride bulk with CEP offered by EUROAPI
Find a price of Glimepiride bulk with CEP offered by EUROAPI
Find a price of Hydrocortisone bulk with JDMF offered by EUROAPI
Find a price of Hydrocortisone Acetate bulk with CEP offered by EUROAPI
Find a price of Hydrocortisone Sodium Succinate bulk with CEP offered by EUROAPI
Find a price of Hydroxocobalamin Acetate bulk with JDMF offered by EUROAPI
Find a price of Hydroxocobalamin Hydrochloride bulk with CEP offered by EUROAPI
Find a price of Hydroxychloroquine Sulphate bulk with DMF offered by EUROAPI
Find a price of Iloprost bulk with DMF offered by EUROAPI
Find a price of Levomepromazine Hydrochloride bulk with JDMF offered by EUROAPI
Find a price of Levomepromazine Maleate bulk with CEP offered by EUROAPI
Find a price of Levomethadone bulk with CEP offered by EUROAPI
Find a price of Limaprost Alfadex bulk with JDMF offered by EUROAPI
Find a price of Morphine Hydrochloride bulk with CEP offered by EUROAPI
Find a price of Morphine Sulfate bulk with CEP offered by EUROAPI
Find a price of Nalbuphine Hydrochloride bulk with DMF offered by EUROAPI
Find a price of Norepinephrine bulk with CEP offered by EUROAPI
Find a price of Norepinephrine Bitartrate bulk with CEP offered by EUROAPI
Find a price of Oxycodone Hydrochloride bulk with CEP offered by EUROAPI
Find a price of Piretanide bulk with CEP offered by EUROAPI
Find a price of Prednicarbate bulk with CEP offered by EUROAPI
Find a price of Prednisolone bulk with CEP offered by EUROAPI
Find a price of Teicoplanin bulk with JDMF offered by EUROAPI
Find a price of Tiaprofenic Acid bulk with CEP offered by EUROAPI
Find a price of Treprostinil Sodium bulk with DMF offered by EUROAPI
Find a price of Triamcinolone Acetonide bulk with DMF offered by EUROAPI
Find a price of Vitamin B12 bulk with CEP offered by EUROAPI
Find a price of Zolpidem Tartrate bulk with JDMF offered by EUROAPI
Find a price of Ramipril bulk offered by EUROAPI
Find a price of Alimemazine bulk offered by EUROAPI
Find a price of Altrenogest bulk offered by EUROAPI
Find a price of Amiodarone Hydrochloride bulk offered by EUROAPI
Find a price of Amisulpride bulk offered by EUROAPI
Find a price of Buprenorphine bulk offered by EUROAPI
Find a price of Buprenorphine Hydrochloride bulk offered by EUROAPI
Find a price of Canrenoate Potassium bulk offered by EUROAPI
Find a price of Chlorpromazine Hydrochloride bulk offered by EUROAPI
Find a price of Clonazepam bulk offered by EUROAPI
Find a price of Cloprostenol Sodium bulk offered by EUROAPI
Find a price of Codeine Sulfate bulk offered by EUROAPI
Find a price of Cyamemazine bulk offered by EUROAPI
Find a price of Cyamemazine Tartrate bulk offered by EUROAPI
Find a price of Dalbavancin bulk offered by EUROAPI
Find a price of Daptomycin bulk offered by EUROAPI
Find a price of Dexamethasone bulk offered by EUROAPI
Find a price of Dinoprost Tromethamine bulk offered by EUROAPI
Find a price of Divalproex Sodium bulk offered by EUROAPI
Find a price of Drotaverine bulk offered by EUROAPI
Find a price of Eflornithine Hydrochloride bulk offered by EUROAPI
Find a price of Enoxaparin Sodium bulk offered by EUROAPI
Find a price of Epinephrine bulk offered by EUROAPI
Find a price of Epinephrine Bitartrate bulk offered by EUROAPI
Find a price of Fexinidazole bulk offered by EUROAPI
Find a price of Fidaxomicin bulk offered by EUROAPI
Find a price of Fluorometholone bulk offered by EUROAPI
Find a price of Fluticasone Furoate bulk offered by EUROAPI
Find a price of Furosemide bulk offered by EUROAPI
Find a price of Hydrocortisone Valerate bulk offered by EUROAPI
Find a price of Hydroxychloroquine Sulphate bulk offered by EUROAPI
Find a price of Inclisiran bulk offered by EUROAPI
Find a price of Insulin bulk offered by EUROAPI
Find a price of Irbesartan bulk offered by EUROAPI
Find a price of Loprazolam Mesilate bulk offered by EUROAPI
Find a price of Lubiprostone bulk offered by EUROAPI
Find a price of Meglumine Antimonate bulk offered by EUROAPI
Find a price of Methylprednisolone Acetate bulk offered by EUROAPI
Find a price of Misoprostol bulk offered by EUROAPI
Find a price of Nadolol bulk offered by EUROAPI
Find a price of Naloxone Hydrochloride bulk offered by EUROAPI
Find a price of Nedocromil Sodium bulk offered by EUROAPI
Find a price of Noscapine bulk offered by EUROAPI
Find a price of Olmesartan Medoxomil bulk offered by EUROAPI
Find a price of Oxymorphone bulk offered by EUROAPI
Find a price of Prednisolone bulk offered by EUROAPI
Find a price of Prednisolone Acetate bulk offered by EUROAPI
Find a price of Prednisolone Sodium Metasulfobenzoate bulk offered by EUROAPI
Find a price of Promethazine Hydrochloride bulk offered by EUROAPI
Find a price of Rifamycin Sodium bulk offered by EUROAPI
Find a price of Rifapentine bulk offered by EUROAPI
Find a price of Riluzole bulk offered by EUROAPI
Find a price of Roxithromycin bulk offered by EUROAPI
Find a price of Sevelamer Carbonate bulk offered by EUROAPI
Find a price of Sodium Cromoglicate bulk offered by EUROAPI
Find a price of Sodium Valproate bulk offered by EUROAPI
Find a price of Tafluprost bulk offered by EUROAPI
Find a price of Trenbolone bulk offered by EUROAPI
Find a price of Treprostinil Diolamine bulk offered by EUROAPI
Find a price of Tulathromycin bulk offered by EUROAPI
Find a price of Vitamin B12 bulk offered by EUROAPI
Find a price of Zopiclone bulk offered by EUROAPI
Find a price of MANUFACTURING OPERATIONS bulk offered by EUROAPI


 EUROAPI
EUROAPI