
Synopsis
Synopsis
0
JDMF
0
EU WC
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Clexane
2. Ardeparin
3. Bemiparin
4. Semuloparin
5. Lovenox
6. Lmwh
7. Certoparin
8. Dalteparin
9. Fraxiparin
10. Nadroparin
11. Nadroparine
12. Clivarin
13. Clivarine
14. Liquaemin
15. Reviparin
16. Alpha-heparin
17. Heparinic Acid
18. Adomiparin
19. Heparinate
20. Multiparin
21. Novoheparin
22. Parnaparin
23. Parvoparin
24. Sandoparin
25. Sublingula
26. Thromboliquine
27. Tinzaparin
28. Arteven
29. Eparina
30. Heparina
31. Heparine
32. Heparinum
33. Hepathrom
34. Liquemin
35. Octaparin
36. Pabyrin
37. Pk-10169
38. Pularin
39. Subeparin
40. Triofiban
41. Fluxum
42. Vetren
43. Heparin Sulfate
44. Hed-heparin
45. Depo-heparin
46. Lipo-hepin
47. Fragmin A
48. Fragmin B
49. Vitrum Ab
50. Eparina [dcit]
51. Heparin [ban]
52. Adomiparin [usan]
53. Cy 216
54. Fr 860
55. Heparin Cy 216
56. Heparine [inn-french]
57. Heparinum [inn-latin]
58. Heparina [inn-spanish]
59. Semuloparin [usan:inn]
60. Unii-1k5kdi46kz
61. Unii-4qw4an84nq
62. Unii-e47c0nf7lv
63. Unii-vl0l558gcb
64. 1k5kdi46kz
65. 4qw4an84nq
66. E47c0nf7lv
67. Unii-p776jq4r2f
68. Unii-v72ot3k19i
69. Vl0l558gcb
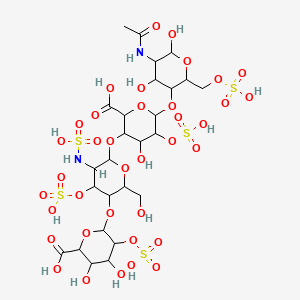
70. 2-o-sulfohexopyranuronosyl-(1->4)-2-deoxy-3-o-sulfo-2-(sulfoamino)hexopyranosyl-(1->4)-2-o-sulfohexopyranuronosyl-(1->4)-2-acetamido-2-deoxy-6-o-sulfohexopyranose
71. Schembl543122
72. Unii-5r0l1d739e
73. Unii-7uq7x4y489
74. Unii-m316wt19d8
75. Unii-s79o08v79f
76. Unii-t2410km04a
77. Gtpl6811
78. M 118reh
79. P776jq4r2f
80. V72ot3k19i
81. Cy 222
82. Hsdb 3094
83. Dtxsid80872762
84. 5r0l1d739e
85. 7uq7x4y489
86. M316wt19d8
87. S79o08v79f
88. T2410km04a
89. Unii-9816xa9004
90. Ave-5026
91. Bcp13334
92. Einecs 232-681-7
93. Kb 101
94. Op 386
95. Op 622
96. M118
97. Rp-54563
98. 9816xa9004
99. M 118
100. Q416516
101. 6-[5-acetamido-4,6-dihydroxy-2-(sulfooxymethyl)oxan-3-yl]oxy-3-[5-(6-carboxy-4,5-dihydroxy-3-sulfooxyoxan-2-yl)oxy-6-(hydroxymethyl)-3-(sulfoamino)-4-sulfooxyoxan-2-yl]oxy-4-hydroxy-5-sulfooxyoxane-2-carboxylic Acid
102. 6-[6-[6-[5-acetamido-4,6-dihydroxy-2-(sulfooxymethyl)tetrahydropyran-3-yl]oxy-2-carboxy-4-hydroxy-5-sulfooxy-tetrahydropyran-3-yl]oxy-2-(hydroxymethyl)-5-(sulfoamino)-4-sulfooxy-tetrahydropyran-3-yl]oxy-3,4-dihydroxy-5-sulfooxy-tetrahydropyran-2-carboxylic Acid
| Molecular Weight | 1134.9 g/mol |
|---|---|
| Molecular Formula | C26H42N2O37S5 |
| XLogP3 | -10.8 |
| Hydrogen Bond Donor Count | 15 |
| Hydrogen Bond Acceptor Count | 38 |
| Rotatable Bond Count | 21 |
| Exact Mass | 1134.0069961 g/mol |
| Monoisotopic Mass | 1134.0069961 g/mol |
| Topological Polar Surface Area | 652 Ų |
| Heavy Atom Count | 70 |
| Formal Charge | 0 |
| Complexity | 2410 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 20 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Lovenox |
| PubMed Health | Enoxaparin (Injection) |
| Drug Classes | Anticoagulant |
| Active Ingredient | Enoxaparin sodium |
| Dosage Form | Injectable |
| Route | Intravenous, subcutaneous; injection |
| Strength | 300mg/3ml (100mg/ml); 100mg/ml |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 2 | |
|---|---|
| Drug Name | Lovenox |
| PubMed Health | Enoxaparin (Injection) |
| Drug Classes | Anticoagulant |
| Active Ingredient | Enoxaparin sodium |
| Dosage Form | Injectable |
| Route | Intravenous, subcutaneous; injection |
| Strength | 300mg/3ml (100mg/ml); 100mg/ml |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
For prevention of deep vein thrombosis, which may result in pulmonary embolism, following knee surgery.
Enoxaparin is indicated for the prevention of ischemic complications in unstable angina and in non Q-wave myocardial infarction; it is indicated in conjunction with percutaneous intervention and/or other treatment for the management of acute ST elevation myocardial infarction. Enoxaparin is also indicated in the prophylaxis of DVT in abdominal surgery, hip replacement, knee replacement, or medical patients with severely restricted mobility during acute illness. Additionally, enoxaparin is indicated for the inpatient treatment of DVT with or without pulmonary embolism and the treatment of outpatient DVT without pulmonary embolism.
Investigated for use/treatment in cardiovascular disorders and coronary artery disease.
Dalteparin is used as a prophylaxis for deep-vein thrombosis and pulmonary embolisms in patients undergoing general surgery (e.g., abdominal, gynecologic, urologic), and in patients with acute medical conditions (e.g. cancer, bed rest, heart failure, severe lung disease). It is also used in patients who have severely restricted mobility, which poses a risk for thromboembolic complications. Dalteparin is also used concomitantly with aspirin and/or other therapy (e.g., nitrates, -adrenergic blockers, clopidogrel, platelet glycoprotein [GP] IIb/IIIa-receptor inhibitors) to reduce the risk of acute cardiac ischemic events. The patients who undergo this treatment combination have unstable angina or non-ST-segment elevation/non-Q-wave myocardial infarction (i.e., non-ST-segment elevation acute coronary syndromes). It is also used in the prevention of clotting during hemodialysis and hemofiltration in connection with acute renal failure or chronic renal insufficiency.
Tinzaparin is used for the prevention of postoperative venous thromboembolism in patients undergoing orthopedic surgery and in patients undergoing general surgery who are at high risk of developing postoperative venous thromboembolism. It is also used for the treatment of deep vein thrombosis and/or pulmonary embolism. It is indicated for use in preventing clot formation in indwelling intravenous lines for hemodialysis.
Nadroparin is used for prophylaxis of thromboembolic disorders and general surgery in orthopedic surgery, treatment of deep vein thrombosis, prevention of clotting during hemodialysis and treatment of unstable angina and non-Q wave myocardial infarction.
Bemiparin is indicated in the following cases: To prevent blood clots in the veins after general abdominal surgery in patients with a moderate risk of venous thromboembolism; in the prevention of the thromboembolic disease in non-surgical patients; prevention of clotting in the extracorporeal circuit during hemodialysis; to prevent blood clots in the veins after a major orthopedic surgery in patients with high risk of venous thromboembolism; secondary prevention of venous thromboembolism; recurrence in patients with deep vein thrombosis; transient prevention and treatment of deep vein thrombosis (DVT).
By the FDA, reviparin is indicated for the treatment of deep vein which may lead to pulmonary embolism in pediatric patients. It is also indicated for the long-term treatment of acute deep vein thrombosis with or without pulmonary embolism in pregnant patients.
Used in the prevention and treatment of venous thromboembolism (deep vein thrombosis and pulmonary embolism) and in the treatment of myocardial infarction.
Used in the prevention and treatment of venous thromboembolism (deep vein thrombosis and pulmonary embolism) and in the treatment of myocardial infarction.
Investigated for use/treatment in thrombosis.
Ardeparin, an anticoagulant, is a fractionated heparin. It acts at multiple sites in the normal coagulation system to inhibit reactions that lead to the clotting of blood and the formation of fibrin clots both in vitro and in vivo.
This drug has an immediate onset of action. Enoxaparin increases Thrombin Time (TT) and activated partial thromboplastin time (aPTT), preventing and reducing thromboembolic complications such as DVT, pulmonary embolism, and ischemic cardiac complications. Administered at 1.5 mg/kg subcutaneously in a pharmacodynamic study, enoxaparin led to a higher ratio of anti-Factor Xa to anti-Factor IIa activity (mean SD, 14.03.1) (based on areas under anti-Factor activity versus time curves) when compared to that of heparin (mean SD, 1.220.13). Increases in the TT and aPTT were 1.8 times those of the control group. Enoxaparin at 1 mg/kg subcutaneously every 12 hours led to aPTT values of 45 seconds or less in most patients. Average aPTT prolongation time on Day 1 was approximately 16% higher than on Day 4 of enoxaparin therapy. Caution is advised during treatment with enoxaparin - the risk of hemorrhage and thrombocytopenia is increased. In pregnant women with prosthetic mechanic heart valves, the risk of thromboembolism is increased.
Dalteparin has an antithrombin binding site that is essential for high affinity binding to the plasma protein antithrombin (ATIII). Anti-Xa activity of plasma is used as both as an estimate of clotting activity, and as a basis to determine dosage. Its use should be avoided in patients with a creatinine clearance less than 20mL/min. In these patients, unfractionated heparin should only be used. As for monitoring, active partial thromboplastin time (aPTT) will only increase at high doses of low molecular weight heparins (LMWH). Therefore, monitoring aPTT is not recommended. However, anti-Xa activity can be measured to monitor the efficacy of the LMWH.
Tinzaparin, like other LMWHs, have a higher anti-Xa activity than anti-IIa activity. The anti-Xa activity of tinzaparin is 2.0 +/- 0.5 times greater than its to anti-IIa activity. Heparin exhibits approximately equal inhibitory activity against Xa and IIa. Tinzaparin is an anticoagulant that blocks the formation of thrombi. Like all LMWHs, tinzaparin only causes activated partial thromboplastin time (aPTT) prolongation at higher doses and routine monitoring is not recommended. However, anti-factor Xa levels may be monitored in some conditions such as pregnancy and renal dysfunction. Its use should be avoided in patients with a creatinine clearance less than 20 mL/min. In these patients, unfractionated heparin should be used. Tinzaparin can be used in patients who have a creatinine clearance between 20-30 mL/min, giving it the highest safety threshold for use in renal failure patients compared to all the LMWHs.
Nadroparin is a low molecular weight heparin that is composed of a heterogeneous mixture of sulfated polysaccaride glycosaminoglycan chains. Th mean molecular weight is approximately 4300 daltons. The ratio of anti-Xa activity to anti-IIa is 3.5:1 whereas it is about 1:1 for heparin. Its use should be avoided in patients with a creatinine clearance less than 40mL/min. In these patients, unfractionated heparin should only be used. As for monitoring, active partial thromboplastin time (aPTT) will only increase at high doses of low molecular weight heparins (LMWH). Therefore, monitoring aPTT is not recommended. However, anti-Xa activity can be measured to monitor the efficacy of the LMWH.
Bemiparin is an anticoagulant classified under the broad category of low molecular weight heparins. In humans, bemiparin has been proven to possess antithrombotic activity and, at therapeutic doses, does not significantly prolong global clotting laboratory tests.
Reviparin is been shown to present significant inhibition of smooth muscle cell migration and proliferation in human cell cultures without affecting endothelial cell growth.
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AB - Heparin group
B01AB04 - Dalteparin
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AB - Heparin group
B01AB05 - Enoxaparin
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AB - Heparin group
B01AB06 - Nadroparin
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AB - Heparin group
B01AB07 - Parnaparin
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AB - Heparin group
B01AB08 - Reviparin
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AB - Heparin group
B01AB10 - Tinzaparin
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AB - Heparin group
B01AB12 - Bemiparin
Absorption
Well absorbed following subcutaneous administration, with a mean bioavailability of 92% (based on anti-factor Xa activity).
Absorption
Mean absolute bioavailability of enoxaparin, after 1-2 mg/kg given subcutaneously is approximately 100% in healthy volunteers. The absorption of enoxaparin is proportional to the dose, demonstrating linear absorption. The average maximum plasma anti-Xa activity is reached 3 to 5 hours after a subcutaneous injection. A 30 mg IV bolus preceding an immediate 1 mg/kg SC every twice a day led to maximum anti-Factor Xa levels of 1.16 IU/mL. Steady-state is reached within 3-4 days of treatment with a Cmax of 1.2 IU/mL. The AUC under the thrombin generation curve was 305 +/- 48.
Route of Elimination
Enoxaparin is mainly excreted by the kidneys. Renal clearance of active fragments represents about 10% of the administered dose and total renal excretion of active and non-active fragments 40% of the dose.
Volume of Distribution
The volume of distribution of enoxaparin is approximately 4-5L, similar to normal blood volume.
Clearance
The mean clearance of enoxaparin is 0.74 L/h after a 1.5 mg/kg intravenous infusion over 6 hours; clearance of enoxaparin is significantly decreased in patients with severe renal impairment.
Absorption
Almost completely absorbed after subcutaneous (sc) doses, with a bioavialability of about 87%.
Route of Elimination
After 4 hours, about 20% is seen in urine. Most of the remainder is found in the liver, gastrointestinal tract and kidney. The kidneys are the major site of dalteparin excretion (approximately 70% based on animal studies).
Volume of Distribution
3 litres
Clearance
Excreted via kidneys. The plasma clearance rate is 33 mL/min.
Absorption
Subcutaneous injection - about 90% when measured as anti-Xa activity versus 67% for anti-IIa activity.
Route of Elimination
Linear elimination through kidneys
Volume of Distribution
Anti-Xa activity is 4 L. Anti-IIa activity is 10.9 L
Clearance
Clearance is dose-dependant. The clearance of tinzaparin based on anti-Xa activity ranged from 1.14 to 2.04 L/hr
Absorption
Absorption is linear. The bioavailability of nadroparin after subcutaneous administration is about 89%.
Route of Elimination
Nadroparin is eliminated via the kidneys through non-saturable mechanisms.
Volume of Distribution
3.59L
Clearance
The clearance of nadroparin is 21.4 +/- 7.0mL/min
Absorption
Hemiparin sodium is rapidly absorbed following its subcutaneous dose of injection, and the bioavailability is estimated to be 96%.
Route of Elimination
This drug is eliminated by the renal and hepatic routes. Elimination is prolonged in those with renal or hepatic impairment.
Volume of Distribution
5.1 L.
Clearance
Elimination occurs in a linear fashion, with a mean clearance time of over 7 h and total clearance of 0.9 L/h.
Liver and the reticulo-endothelial system are the sites of biotransformation.
Enoxaparin is mainly metabolized by the liver via desulfation and/or depolymerization to lower and less potent molecular weight metabolites.
Liver and the reticulo-endothelial system are the sites of biotransformation. They are partially metabolized by desulphatation and depolymerization.
Sulfation and polymerization occurs in the liver.
Nadroparin is metabolized in the liver.
In a study of healthy volunteers, bemiparin 3500 IU achieved more anti-Xa activity than enoxaparin 4000 IU, measured by the area under the curve. The peak of anti-Xa activity was reached at 3h post-administration, and there were anti-Xa measurable levels up to 16 h after subcutaneous injection.
Elimination half-life for anti-factor Xa activity averages 3.3 hours following a single intravenous dose, while elimination half-life for anti-factor IIa activity averages 1.2 hours following a single intravenous dose.
The half-life of enoxaparin is about 4 hours after a single dose administered subcutaneously and about 7 hours after several doses. One source mentions a half-life ranging from 1 hour to 4.5 hours.
Terminal Half life: Intravenous - 2 hours. Subcutaneous - 3-5hours
Anti-Xa activity is 82 minutes. Anti-IIa activity is 71 minutes.
In healthy patients, the half life is between 3.5hrs to 11.2hrs following subcutaneous administration.
Bemiparin, when administered in the dose range of 2,500 IU to 12,500 (therapeutic dosing), it has an approximate half-life of 5-6 hours.
Ardeparin binds to antithrombin III, accelerating its activity in inactivating factor Xa and thrombin, thereby inhibiting thrombosis. Ardeparin also binds to heparin cofactor II, inhibiting thrombin. Ardeparin does not effect prothrombin time (PT) assays and may prolong activated partial thromboplastin time (APTT). Ardeparin has double the anti-factor Xa activity versus anti-factor IIa activity, compared to unfractionated heparin which has approximately equal anti-factor Xa activity and anti-factor IIa activity.
Enoxaparin binds to antithrombin III, a serine protease inhibitor, forming a complex that irreversibly inactivates factor Xa, which is frequently used to monitor anticoagulation in the clinical setting. Following factor Xa inactivation, enoxaparin is released and binds to other anti-thrombin molecules. Factor IIa (thrombin) is directly inhibited by enoxaparin, however with less potency than unfractionated heparin (UFH). Due to the cascade of effects resulting from enoxaparin binding, thrombin is unable to convert fibrinogen to fibrin and form a clot, preventing thromboembolic events.
M118 is a novel drug candidate that has been, through Momenta's proprietary technology, rationally engineered with attributes of monitorability, reversibility, flexible administration (intravenous and subcutaneous), and both potent and predictable anti-Xa (aXa) and anti-IIa (aIIa) activity to provide anticoagulant therapy to patients with ACS. M118 is designed to interact at multiple points in the coagulation cascade by selectively binding to anti-thrombin III and thrombin, two critical factors in the formation of clots.
Dalteparin potentiates the activity of ATIII, inhibiting the formation of both factor Xa and thrombin. The main difference between dalteparin and unfractionated heparin (UH) is that dalteparin preferentially inactivates factor Xa. As a result, only a slight increase in clotting time [(i.e. activated partial thomboplastin time (APTT)] is observed relative to UH. For this same reason, APTT is not used to monitor the effects of dalteparin except as an indicator for overdosage.
Tinzaparin binds to the plasma protein antithrombin III, forming a complex with then accelerates the inhibition of factor Xa. Its affinity for factor Xa is 2-4 times greater than that of unbound ATIII. The inactivation of factor Xa in turn will exponentially generation of thrombin (factor IIa) molecules, which is needed to activate fibrinogen to fibrin. The coagulation cascade is inhibited because fibrin cannot be formed in the presence of tinzaparin. Like all LMWH, it cannot be given intramuscularly due to increased risk of hematoma.
The mechanism of action for nadroparin is similar to all other LMWHs. Like all LMWHs, nadroparin has a pentasaccharide sequence which binds to ATIII, which potentiates the action of ATIII. This complex greatly accelerates the inactivation of factor Xa and factor IIa. As a result, the coagulation cascade is inhibited.
This drug is a second-generation low molecular weight heparin (LMWH). It has a very low mean molecular weight (3600 Dalton), a long half-life (5.3 hrs) and a large anti-Xa: anti-IIa ratio (8:1). The mechanism of action of bemiparin is inhibition of factor Xa, which is a necessary step in the clotting cascade. Factor-Xa is necessary for the propagation of a thrombus. Combined with various co-factors that bind to activated platelets, Factor-Xa increases coagulation by converting prothrombin to thrombin. Activated Factor-X, bound as part of the prothrombinase complex on the external surface of activated platelets, converts significant amounts of prothrombin to thrombin, promoting the so-called thrombin burst, referring to a burst of thrombin release. A secondary but less potent mechanism of action of this drug is binding to antithrombin III and activated factor II (Factor IIa), which further prevents the propagation of thrombi. Due to its excellent pharmacological profile-the second-generation LMWH with the lowest molecular weight, the longest half-life and the highest anti-Factor Xa/anti-Factor IIa activity ratio-it can be safely used in special categories of patients (children, elderly, patients with renal impairment and congestive heart failure). Several studies demonstrated its safety and efficacy, while cost analyses show the economic benefits of bemiparin treatment as compared to other heparins.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
20
PharmaCompass offers a list of Enoxaparin Sodium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Enoxaparin Sodium manufacturer or Enoxaparin Sodium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Enoxaparin Sodium manufacturer or Enoxaparin Sodium supplier.
PharmaCompass also assists you with knowing the Enoxaparin Sodium API Price utilized in the formulation of products. Enoxaparin Sodium API Price is not always fixed or binding as the Enoxaparin Sodium Price is obtained through a variety of data sources. The Enoxaparin Sodium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Enoxaparin Sodium manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Enoxaparin Sodium, including repackagers and relabelers. The FDA regulates Enoxaparin Sodium manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Enoxaparin Sodium API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Enoxaparin Sodium manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Enoxaparin Sodium supplier is an individual or a company that provides Enoxaparin Sodium active pharmaceutical ingredient (API) or Enoxaparin Sodium finished formulations upon request. The Enoxaparin Sodium suppliers may include Enoxaparin Sodium API manufacturers, exporters, distributors and traders.
click here to find a list of Enoxaparin Sodium suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Enoxaparin Sodium DMF (Drug Master File) is a document detailing the whole manufacturing process of Enoxaparin Sodium active pharmaceutical ingredient (API) in detail. Different forms of Enoxaparin Sodium DMFs exist exist since differing nations have different regulations, such as Enoxaparin Sodium USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Enoxaparin Sodium DMF submitted to regulatory agencies in the US is known as a USDMF. Enoxaparin Sodium USDMF includes data on Enoxaparin Sodium's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Enoxaparin Sodium USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Enoxaparin Sodium suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Enoxaparin Sodium Drug Master File in Korea (Enoxaparin Sodium KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Enoxaparin Sodium. The MFDS reviews the Enoxaparin Sodium KDMF as part of the drug registration process and uses the information provided in the Enoxaparin Sodium KDMF to evaluate the safety and efficacy of the drug.
After submitting a Enoxaparin Sodium KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Enoxaparin Sodium API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Enoxaparin Sodium suppliers with KDMF on PharmaCompass.
A Enoxaparin Sodium CEP of the European Pharmacopoeia monograph is often referred to as a Enoxaparin Sodium Certificate of Suitability (COS). The purpose of a Enoxaparin Sodium CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Enoxaparin Sodium EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Enoxaparin Sodium to their clients by showing that a Enoxaparin Sodium CEP has been issued for it. The manufacturer submits a Enoxaparin Sodium CEP (COS) as part of the market authorization procedure, and it takes on the role of a Enoxaparin Sodium CEP holder for the record. Additionally, the data presented in the Enoxaparin Sodium CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Enoxaparin Sodium DMF.
A Enoxaparin Sodium CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Enoxaparin Sodium CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Enoxaparin Sodium suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Enoxaparin Sodium as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Enoxaparin Sodium API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Enoxaparin Sodium as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Enoxaparin Sodium and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Enoxaparin Sodium NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Enoxaparin Sodium suppliers with NDC on PharmaCompass.
Enoxaparin Sodium Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Enoxaparin Sodium GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Enoxaparin Sodium GMP manufacturer or Enoxaparin Sodium GMP API supplier for your needs.
A Enoxaparin Sodium CoA (Certificate of Analysis) is a formal document that attests to Enoxaparin Sodium's compliance with Enoxaparin Sodium specifications and serves as a tool for batch-level quality control.
Enoxaparin Sodium CoA mostly includes findings from lab analyses of a specific batch. For each Enoxaparin Sodium CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Enoxaparin Sodium may be tested according to a variety of international standards, such as European Pharmacopoeia (Enoxaparin Sodium EP), Enoxaparin Sodium JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Enoxaparin Sodium USP).

