06 Feb 2026
// PHARMIWEB
08 Jan 2026
// PRESS RELASE
25 Nov 2025
// BUSINESS STD
Latest Content by PharmaCompass

 KEY PRODUCTS
KEY PRODUCTS

![]() Reset all filters
Reset all filters
01 1Aceclidine Hydrochloride
02 1Acoltremon
03 1Acoramidis Hydrochloride
04 2Apixaban
05 1Apremilast
06 4Aripiprazole
07 1Aripiprazole Lauroxil
08 4Brinzolamide
09 8CAS 120011-70-3
10 1Canagliflozin
11 2Ciprofloxacin
12 2Ciprofloxacin Hydrochloride
13 2Dabigatran Etexilate Mesylate
14 1Dapagliflozin
15 1Dapagliflozin Propanediol Monohydrate
16 1Deferasirox
17 1Desvenlafaxine Succinate
18 1Difelikefalin Acetate
19 11Donepezil
20 2Dorzolamide Hydrochloride
21 1Edaravone
22 1Edoxaban Tosylate
23 1Elagolix Sodium
24 1Empagliflozin
25 2Enalapril Maleate
26 1Ensifentrine
27 2Entacapone
28 1Escitalopram Oxalate
29 2Ezetimibe
30 1Febuxostat
31 1Fexuprazan
32 1Fezolinetant
33 1Indacaterol Maleate
34 4Ipratropium Bromide
35 1Ivacaftor
36 1Labetalol Hydrochloride
37 2Levetiracetam
38 1Levofloxacin
39 1Levofloxacin Hemihydrate
40 1Linaclotide
41 2Linezolid
42 1Mirabegron
43 1Mirogabalin Besylate
44 5Mirtazapine
45 1Moxonidine
46 3Paliperidone Palmitate
47 1Posaconazole
48 1Propofol
49 1Resmetirom
50 2Rivaroxaban
51 1Ropinirole
52 1Ropinirole Hydrochloride
53 1Rotigotine
54 2Salbutamol Sulphate
55 1Salmeterol Xinafoate
56 1Sotalol Hydrochloride
57 1Sugammadex Sodium
58 1Suzetrigine
59 1Tafamidis Meglumine
60 1Tegoprazan
61 1Ticagrelor
62 1Tirzepatide
63 1Tolvaptan
64 1Trospium Chloride
65 1Vibegron
66 1Vilanterol Trifenatate
67 1Vonoprazan Fumarate
68 1Vortioxetine Hydrobromide

![]() Reset all filters
Reset all filters
01 57Active
02 55Blank
01 5Valid
02 107Blank

![]() Reset all filters
Reset all filters
01 1219MF10252
02 1219MF10357
03 1301MF10005
04 1302MF10084
05 1307MF10136
06 107Blank

![]() Reset all filters
Reset all filters
01 24WC-0036
02 20WC-0037
03 2WC-0475
04 2WC-0478
05 2WC-478
06 62Blank

![]() Reset all filters
Reset all filters
01 120050831-12-B-44-02
02 120050831-12-B-44-02(1)
03 120080507-85-D-53-09
04 120080507-85-D-53-09(1)
05 120130614-188-I-356-02
06 120130614-188-I-356-02(1)
07 120130614-188-I-356-02(2)
08 120130614-188-I-356-02(A)
09 120141008-158-I-439-23
10 120141008-158-I-439-23(1)
11 120141008-158-I-439-23(11)
12 120141008-158-I-439-23(13)
13 120141008-158-I-439-23(14)
14 120141008-158-I-439-23(2)
15 120141008-158-I-439-23(4)
16 120141008-158-I-439-23(5)
17 120141008-158-I-439-23(6)
18 120141008-158-I-439-23(7)
19 120141008-158-I-439-23(8)
20 120141008-158-I-439-23(9)
21 120141008-158-I-439-23(A)
22 120150618-158-I-428-25
23 120150618-158-I-428-25(1)
24 120150618-158-I-428-25(2)
25 120150618-158-I-428-25(3)
26 120150618-158-I-428-25(5)
27 120150618-158-I-428-25(A)
28 120160610-183-I-484-26
29 120180110-209-J-10
30 120180110-209-J-10(1)
31 120200707-209-J-678
32 120201022-209-J-571
33 120201022-209-J-571(1)
34 120201022-209-J-571(2)
35 120201022-209-J-571(3)
36 120210113-209-J-520
37 120210325-210-J-630
38 120210511-209-J-985
39 120230420-209-J-1481
40 120230919-209-J-1546
41 1Su434-11-ND
42 1Su434-11-ND(1)
43 1Su434-12-ND
44 1Su434-25-ND
45 1Su434-42-ND
46 1Su4579-6-ND
47 1Su4579-6-ND(1)
48 65Blank

![]() Reset all filters
Reset all filters
01 158032-0002
02 158032-0100
03 158032-0121
04 158032-0125
05 158032-0132
06 158032-0133
07 158032-0134
08 158032-0137
09 158032-0142
10 158032-0143
11 158032-0803
12 158032-1005
13 158032-1006
14 158032-1010
15 158032-1011
16 158032-1017
17 158032-1020
18 158032-1022
19 158032-1025
20 158032-1026
21 158032-1028
22 158032-2003
23 158032-2004
24 158032-2005
25 158032-2009
26 158032-2011
27 158032-2017
28 158032-2019
29 158032-2021
30 158032-2023
31 158032-2025
32 158032-2027
33 158032-2028
34 158032-2029
35 158032-2030
36 158032-2031
37 158032-2032
38 158032-2035
39 158032-2037
40 158032-2038
41 158032-2040
42 158032-3001
43 70Blank
01 112Blank

 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2013-04-02
Pay. Date : 2013-09-27
DMF Number : 18465
Submission : 2005-06-24
Status : Active
Type : II

Registration Number : 307MF10136
Registrant's Address : 11th Floor (5th Level), Phoenix IVY Building, Plot No. 573A-Ⅲ, Road No. 82, Jubilee Hills, Hyderabad-500033 Telangana, India
Initial Date of Registration : 2025-11-05
Latest Date of Registration :

Date of Issue : 2025-06-27
Valid Till : 2028-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-2027
Start Marketing Date : 2017-12-14
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2012-10-26
Pay. Date : 2012-12-13
DMF Number : 13250
Submission : 1998-09-29
Status : Active
Type : II

Certificate Number : CEP 2000-405 - Rev 13
Issue Date : 2024-10-16
Type : Chemical
Substance Number : 888
Status : Valid

Date of Issue : 2025-06-27
Valid Till : 2028-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-3001
Start Marketing Date : 2023-03-17
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Seongwoo Chemical Co., Ltd.
Registration Date : 2019-01-31
Registration Number : 20080507-85-D-53-09(1)
Manufacturer Name : Neuland Laboratories Ltd
Manufacturer Address : Plot No.s 92,93,94,257,258,259 IDA, Pashamylaram, Isnapur, Patancheru(M), Medak Dist. -502 319, Andhra Pradesh, India


GDUFA
DMF Review : Reviewed
Rev. Date : 2016-04-15
Pay. Date : 2016-03-17
DMF Number : 25733
Submission : 2012-01-26
Status : Active
Type : II

Certificate Number : CEP 2020-280 - Rev 02
Issue Date : 2024-03-20
Type : Chemical
Substance Number : 2933
Status : Valid

Date of Issue : 2025-06-27
Valid Till : 2028-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-2017
Start Marketing Date : 2017-12-14
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Aging Life Science Co., Ltd.
Registration Date : 2020-06-30
Registration Number : Su434-12-ND
Manufacturer Name : Neuland Laboratories Limited (Unit-II)
Manufacturer Address : Plot No. 92-94, 257-259 IDA, Pashamylaram, Isnapur Village, Patancheru Mandal, Sangareddy District - 502319 Telangana State, India


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15434
Submission : 2001-05-15
Status : Active
Type : II

Registration Number : 219MF10252
Registrant's Address : 11th Floor (5th Office Level), Phoenix IVY Building, Plot No. 573A-Ⅲ, Road No. 82, Jubilee Hills,Hyderabad-33 Telangana, India
Initial Date of Registration : 2007-07-26
Latest Date of Registration :

Date of Issue : 2025-06-16
Valid Till : 2028-06-16
Written Confirmation Number : WC-0036
Address of the Firm :

NDC Package Code : 58032-0125
Start Marketing Date : 2017-11-27
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Hwail Pharmaceutical Co., Ltd.
Registration Date : 2018-10-14
Registration Number : 20050831-12-B-44-02(1)
Manufacturer Name : Neuland Lab. Ltd
Manufacturer Address : Sy.No: 347,473,474,490/2 Bonthapalli (V), Veerabhadraswamy temple road, Jinnaram (M), Medak (Dist.)-502 313 Telangana, India


GDUFA
DMF Review : Reviewed
Rev. Date : 2017-01-31
Pay. Date : 2017-01-11
DMF Number : 30564
Submission : 2016-06-07
Status : Active
Type : II

Certificate Number : CEP 2016-222 - Rev 04
Issue Date : 2024-05-07
Type : Chemical
Substance Number : 923
Status : Valid

Date of Issue : 2025-06-27
Valid Till : 2028-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-2025
Start Marketing Date : 2016-07-09
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2015-05-04
Pay. Date : 2014-07-24
DMF Number : 17799
Submission : 2004-11-02
Status : Active
Type : II

Registration Number : 302MF10084
Registrant's Address : 11th Floor (5th Level), Phoenix IVY Building, Plot No. 573A-Ⅲ, Road No. 82, Jubilee Hills,Hyderabad-500033 Telangana, India
Initial Date of Registration : 2020-07-16
Latest Date of Registration :

Date of Issue : 2025-06-16
Valid Till : 2028-06-16
Written Confirmation Number : WC-0036
Address of the Firm :

NDC Package Code : 58032-0132
Start Marketing Date : 2020-03-06
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Korea Pfizer Pharmaceutical Co., Ltd.
Registration Date : 2016-06-10
Registration Number : 20160610-183-I-484-26
Manufacturer Name : Neuland Laboratories Limited (Unit-Ⅰ)
Manufacturer Address : Sy. No. 347, 473, 474, 490/2, Bonthapalli Village, Veerabhadra swamy Temple Road, Jinnaram Mandal, Medak District - 502 313, Telangana State, India


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16007
Submission : 2002-06-11
Status : Active
Type : II

Registration Number : 301MF10005
Registrant's Address : 11th Floor (5th Office Level), Phoenix IVY Building, Plot No. 573A-Ⅲ, Road No. 82, Jubilee Hills,Hyderabad-33 Telangana, India
Initial Date of Registration : 2019-05-15
Latest Date of Registration :

Date of Issue : 2025-06-27
Valid Till : 2028-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-1005
Start Marketing Date : 2017-12-22
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Medipoem Co., Ltd.
Registration Date : 2024-01-12
Registration Number : 20201022-209-J-571(3)
Manufacturer Name : Neuland Laboratories Limited
Manufacturer Address : Unit-I, Sy. No. 347,473,474, 490/2, Bonthapally Village, Veerabhadraswamy Temple road, Gummadidala Mandal, Sangareddy District-502 313, Telangana State, India


GDUFA
DMF Review : Reviewed
Rev. Date : 2018-07-16
Pay. Date : 2018-06-13
DMF Number : 32422
Submission : 2018-01-31
Status : Active
Type : II

Date of Issue : 2025-06-27
Valid Till : 2028-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-2028
Start Marketing Date : 2017-12-13
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Aging Life Science Co., Ltd.
Registration Date : 2021-11-04
Registration Number : Su434-42-ND
Manufacturer Name : Neuland Laboratories Limited
Manufacturer Address : Plot No. 92-94, 257-259 IDA, Pashamylaram, Isnapur Village, Patancheru Mandal, Sangareddy District - 502319, Telangana State, India

| Available Reg. Filing : ASMF |


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 32953
Submission : 2018-11-06
Status : Active
Type : II

Date of Issue : 2025-06-27
Valid Till : 2028-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-2030
Start Marketing Date : 2018-11-14
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Aging Life Science Co., Ltd.
Registration Date : 2020-12-17
Registration Number : Su434-25-ND
Manufacturer Name : Neuland Laboratories Limited (Unit-II)
Manufacturer Address : Plot Nos. 92, 93, 94, 257, 258, 259 IDA, Pashamylaram, Isnapur Village, Patancheru Mandal, Sangareddy District – 502 319, Telangana State India

| Available Reg. Filing : ASMF |


GDUFA
DMF Review : Reviewed
Rev. Date : 2021-11-18
Pay. Date : 2021-08-10
DMF Number : 22889
Submission : 2009-06-24
Status : Active
Type : II

Date of Issue : 2025-06-27
Valid Till : 2028-06-16
Written Confirmation Number : WC-0037
Address of the Firm :

NDC Package Code : 58032-2005
Start Marketing Date : 2017-12-14
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg. Filing : ASMF |

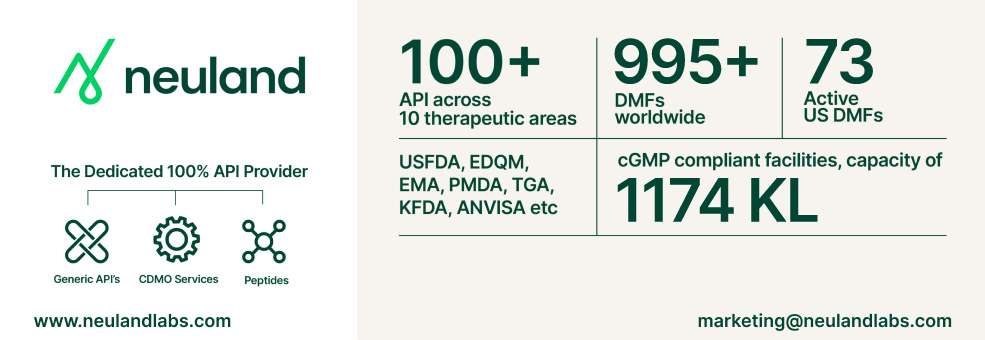
Neuland Laboratories is a supplier offers 88 products (APIs, Excipients or Intermediates).
Find a price of Ciprofloxacin bulk with DMF, JDMF, WC offered by Neuland Laboratories
Find a price of Ciprofloxacin Hydrochloride bulk with DMF, CEP, WC offered by Neuland Laboratories
Find a price of Deferasirox bulk with DMF, CEP, WC offered by Neuland Laboratories
Find a price of Enalapril Maleate bulk with DMF, JDMF, WC offered by Neuland Laboratories
Find a price of Labetalol Hydrochloride bulk with DMF, CEP, WC offered by Neuland Laboratories
Find a price of Levetiracetam bulk with DMF, JDMF, WC offered by Neuland Laboratories
Find a price of Mirtazapine bulk with DMF, JDMF, WC offered by Neuland Laboratories
Find a price of Apixaban bulk with DMF, WC offered by Neuland Laboratories
Find a price of Apremilast bulk with DMF, WC offered by Neuland Laboratories
Find a price of Aripiprazole bulk with DMF, WC offered by Neuland Laboratories
Find a price of Brinzolamide bulk with DMF, WC offered by Neuland Laboratories
Find a price of Dabigatran Etexilate Mesylate bulk with DMF, WC offered by Neuland Laboratories
Find a price of Dapagliflozin Propanediol Monohydrate bulk with DMF, WC offered by Neuland Laboratories
Find a price of Donepezil bulk with DMF, WC offered by Neuland Laboratories
Find a price of Dorzolamide Hydrochloride bulk with DMF, WC offered by Neuland Laboratories
Find a price of Edaravone bulk with DMF, WC offered by Neuland Laboratories
Find a price of Elagolix Sodium bulk with DMF, WC offered by Neuland Laboratories
Find a price of Entacapone bulk with DMF, WC offered by Neuland Laboratories
Find a price of Escitalopram Oxalate bulk with DMF, WC offered by Neuland Laboratories
Find a price of Ezetimibe bulk with DMF, WC offered by Neuland Laboratories
Find a price of Indacaterol Maleate bulk with DMF, WC offered by Neuland Laboratories
Find a price of Ipratropium Bromide bulk with DMF, WC offered by Neuland Laboratories
Find a price of Linezolid bulk with DMF, WC offered by Neuland Laboratories
Find a price of Mirabegron bulk with DMF, CEP offered by Neuland Laboratories
Find a price of Mirtazapine bulk with DMF, WC offered by Neuland Laboratories
Find a price of Moxonidine bulk with CEP, WC offered by Neuland Laboratories
Find a price of Paliperidone Palmitate bulk with DMF, WC offered by Neuland Laboratories
Find a price of Posaconazole bulk with DMF, WC offered by Neuland Laboratories
Find a price of Propofol bulk with DMF, WC offered by Neuland Laboratories
Find a price of Rivaroxaban bulk with DMF, WC offered by Neuland Laboratories
Find a price of Ropinirole bulk with DMF, WC offered by Neuland Laboratories
Find a price of Ropinirole Hydrochloride bulk with DMF, WC offered by Neuland Laboratories
Find a price of Rotigotine bulk with DMF, WC offered by Neuland Laboratories
Find a price of Salbutamol Sulphate bulk with DMF, WC offered by Neuland Laboratories
Find a price of Salmeterol Xinafoate bulk with DMF, WC offered by Neuland Laboratories
Find a price of Sotalol Hydrochloride bulk with DMF, WC offered by Neuland Laboratories
Find a price of Sugammadex Sodium bulk with DMF, WC offered by Neuland Laboratories
Find a price of Tafamidis Meglumine bulk with DMF, WC offered by Neuland Laboratories
Find a price of Ticagrelor bulk with DMF, WC offered by Neuland Laboratories
Find a price of Vilanterol Trifenatate bulk with DMF, WC offered by Neuland Laboratories
Find a price of Apixaban bulk with DMF offered by Neuland Laboratories
Find a price of Aripiprazole bulk with WC offered by Neuland Laboratories
Find a price of Aripiprazole Lauroxil bulk with DMF offered by Neuland Laboratories
Find a price of Brinzolamide bulk with DMF offered by Neuland Laboratories
Find a price of Ciprofloxacin bulk with JDMF offered by Neuland Laboratories
Find a price of Dabigatran Etexilate Mesylate bulk with DMF offered by Neuland Laboratories
Find a price of Difelikefalin Acetate bulk with DMF offered by Neuland Laboratories
Find a price of Donepezil bulk with WC offered by Neuland Laboratories
Find a price of Dorzolamide Hydrochloride bulk with DMF offered by Neuland Laboratories
Find a price of Empagliflozin bulk with DMF offered by Neuland Laboratories
Find a price of Enalapril Maleate bulk with DMF offered by Neuland Laboratories
Find a price of Levofloxacin bulk with DMF offered by Neuland Laboratories
Find a price of Levofloxacin Hemihydrate bulk with WC offered by Neuland Laboratories
Find a price of Linezolid bulk with DMF offered by Neuland Laboratories
Find a price of Mirtazapine bulk with DMF offered by Neuland Laboratories
Find a price of Paliperidone Palmitate bulk with WC offered by Neuland Laboratories
Find a price of Vonoprazan Fumarate bulk with DMF offered by Neuland Laboratories
Find a price of Ciprofloxacin Hydrochloride bulk offered by Neuland Laboratories
Find a price of Levetiracetam bulk offered by Neuland Laboratories
Find a price of Aceclidine Hydrochloride bulk offered by Neuland Laboratories
Find a price of Acoltremon bulk offered by Neuland Laboratories
Find a price of Acoramidis Hydrochloride bulk offered by Neuland Laboratories
Find a price of Canagliflozin bulk offered by Neuland Laboratories
Find a price of CAS 120011-70-3 bulk offered by Neuland Laboratories
Find a price of Dapagliflozin bulk offered by Neuland Laboratories
Find a price of Desvenlafaxine Succinate bulk offered by Neuland Laboratories
Find a price of Donepezil bulk offered by Neuland Laboratories
Find a price of Edoxaban Tosylate bulk offered by Neuland Laboratories
Find a price of Ensifentrine bulk offered by Neuland Laboratories
Find a price of Entacapone bulk offered by Neuland Laboratories
Find a price of Ezetimibe bulk offered by Neuland Laboratories
Find a price of Febuxostat bulk offered by Neuland Laboratories
Find a price of Fexuprazan bulk offered by Neuland Laboratories
Find a price of Fezolinetant bulk offered by Neuland Laboratories
Find a price of Ipratropium Bromide bulk offered by Neuland Laboratories
Find a price of Ivacaftor bulk offered by Neuland Laboratories
Find a price of Linaclotide bulk offered by Neuland Laboratories
Find a price of Mirogabalin Besylate bulk offered by Neuland Laboratories
Find a price of Mirtazapine bulk offered by Neuland Laboratories
Find a price of Resmetirom bulk offered by Neuland Laboratories
Find a price of Rivaroxaban bulk offered by Neuland Laboratories
Find a price of Suzetrigine bulk offered by Neuland Laboratories
Find a price of Tegoprazan bulk offered by Neuland Laboratories
Find a price of Tirzepatide bulk offered by Neuland Laboratories
Find a price of Tolvaptan bulk offered by Neuland Laboratories
Find a price of Trospium Chloride bulk offered by Neuland Laboratories
Find a price of Vibegron bulk offered by Neuland Laboratories
Find a price of Vortioxetine Hydrobromide bulk offered by Neuland Laboratories