
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
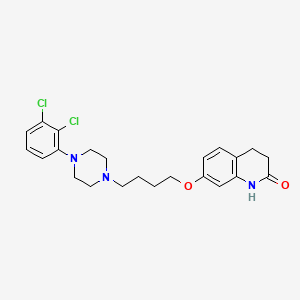

1. 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butyloxy)-3,4-dihydro-2(1h)-quinolinone
2. Abilify
3. Aripiprazol
4. Opc 14597
5. Opc-14597
1. 129722-12-9
2. Abilify
3. Abilitat
4. Opc-14597
5. Abilify Discmelt
6. Opc 14597
7. Opc 31
8. Aripiprex
9. Abilify Maintena
10. Abilify Mycite
11. Opc-31
12. 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1h-quinolin-2-one
13. 7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1h)-one
14. 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril
15. Nsc-759266
16. Chembl1112
17. 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydrocarbostyril
18. 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydro-2(1h)-quinolinone
19. 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-2(1h)-quinolinone
20. 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1h)-one
21. Aripiprazol
22. Discmelt
23. Chebi:31236
24. 82vfr53i78
25. 129722-12-9 (free Base)
26. Mfcd00892072
27. 2(1h)-quinolinone, 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-
28. 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-1,2,3,4-tetrahydroquinolin-2-one
29. Ncgc00159510-02
30. Aripiprazole [usan]
31. 2(1h)-quinolinone, 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydro-
32. Dsstox_cid_26083
33. Dsstox_rid_81324
34. Dsstox_gsid_46083
35. Aripiprazolum
36. 7-[4-[4-[2,3-bis(chloranyl)phenyl]piperazin-1-yl]butoxy]-3,4-dihydro-1h-quinolin-2-one
37. 7-{4-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-butoxy}-3,4-dihydro-1h-quinol In-2-one
38. Smr000466383
39. Abilify (tn)
40. Abilify Maintena Kit
41. Cas-129722-12-9
42. Hsdb 7320
43. Sr-01000759353
44. Unii-82vfr53i78
45. Abilify Digital
46. 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butyloxy)-3,4-dihydro-2(1h)-quinolinone
47. Aripiprazole [usan:inn:ban]
48. 7-(4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy)-3,4-dihydrocarbostyril
49. 7-{4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy}-3,4-dihydrocarbostyril
50. Abilify Od
51. 7-(4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy)-3,4-dihydroquinolin-2(1h)-one
52. Aripiprazole Depot
53. 9sc
54. Aripiprazole Solution
55. Aripiprazole- Bio-x
56. Bms-337039
57. Ks-1030
58. Aripiprazole (abilify)
59. Cpd000466383
60. Aripiprazole-d8 Solution
61. Abilify Mycite Kit
62. Aripiprazole [mi]
63. Aripiprazole [inn]
64. Aripiprazole [jan]
65. Gtpl34
66. Aripiprazole [hsdb]
67. Aripiprazole-d8(butyl-d8)
68. Schembl8255
69. Aripiprazole [vandf]
70. Aripiprazole [mart.]
71. Mls000759517
72. Mls001165779
73. Mls001195621
74. Mls001424078
75. Mls006011892
76. Aripiprazole [usp-rs]
77. Aripiprazole [who-dd]
78. Aripiprazole (jan/usp/inn)
79. Schembl5335696
80. Aripiprazole [ema Epar]
81. Aristada (aripiprazole Lauroxil)
82. Dtxsid3046083
83. Schembl12961254
84. Aripiprazole [orange Book]
85. Hms2051i18
86. Hms2089m20
87. Hms2093f22
88. Hms2230m18
89. Hms3373d04
90. Hms3393i18
91. Hms3657k13
92. Hms3715j07
93. Hms3744a13
94. Hms3884e18
95. Pharmakon1600-01505851
96. Aripiprazole [ep Monograph]
97. Act03221
98. Bcp04902
99. Zinc1851149
100. Aripiprazole [usp Monograph]
101. Tox21_111728
102. Bbl029082
103. Bdbm50130293
104. Dl-178
105. Nsc759266
106. S1975
107. Stk625160
108. Akos005558247
109. Tox21_111728_1
110. Ab07660
111. Ac-1554
112. Bcp9000317
113. Ccg-100891
114. Cs-0766
115. Db01238
116. Nc00141
117. Nsc 759266
118. Aripiprazole 1.0 Mg/ml In Acetonitrile
119. Ncgc00159510-03
120. Ncgc00159510-04
121. Ncgc00159510-05
122. Ba164218
123. Hy-14546
124. Sy053146
125. Sbi-0206870.p001
126. Am20090766
127. Ft-0600352
128. Ft-0662278
129. Ft-0662279
130. Sw197521-3
131. A19454
132. D01164
133. Ab00639935-09
134. Ab00639935_10
135. Ab00639935_11
136. 722a129
137. L001339
138. Q411188
139. J-005707
140. Sr-01000759353-4
141. Sr-01000759353-6
142. Brd-k70358946-001-06-6
143. Z1541632800
144. 7-[4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy]-3,4-dihydro-2(1h)-quinolinone
145. 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]3,4-dihydro-2(1h)-quinolinone
146. 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-quinolin-2(1h)-one
147. 7-{4-[-4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy}-3,4-dihydrocarbostyril
148. 7-{4-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-butoxy}-3,4-dihydro-1h-quinolin-2-one
149. 7-{4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy}-3, 4-dihydrocarbostyril
150. 7-{4-[4-(2,3-dichlorophenyl)piperazino]butoxy}-3,4-dihydro-2(1h)-quinolinone
151. Aripiprazole; 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydrocarbostyril
152. 1026778-41-5
153. 24-29-3
154. Aripiprazole Solution, 1.0 Mg/ml (50:50 Methanol/water With 1% 1n Hcl), Ampule Of 1 Ml, Certified Reference Material
155. Aripiprazole Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 448.4 g/mol |
|---|---|
| Molecular Formula | C23H27Cl2N3O2 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 447.1480325 g/mol |
| Monoisotopic Mass | 447.1480325 g/mol |
| Topological Polar Surface Area | 44.8 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 559 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Abilify |
| PubMed Health | Aripiprazole (Injection) |
| Drug Classes | Antipsychotic |
| Drug Label | Aripiprazole is a psychotropic drug that is available as ABILIFY (aripiprazole) Tablets, ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets, ABILIFY (aripiprazole) Oral Solution, and ABILIFY (aripiprazole) Injection, a solution for... |
| Active Ingredient | Aripiprazole |
| Dosage Form | Solution; Injectable; Tablet, orally disintegrating; Tablet |
| Route | Intramuscular; Oral |
| Strength | 30mg; 10mg; 5mg; 9.75mg/1.3ml (7.5mg/ml); 15mg; 1mg/ml; 20mg; 2mg |
| Market Status | Prescription |
| Company | Otsuka |
| 2 of 4 | |
|---|---|
| Drug Name | Aripiprazole |
| PubMed Health | Aripiprazole |
| Drug Classes | Antipsychotic |
| Drug Label | Aripiprazole is a psychotropic drug that is available as ABILIFY (aripiprazole) Tablets, ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets, ABILIFY (aripiprazole) Oral Solution, and ABILIFY (aripiprazole) Injection, a solution for... |
| Active Ingredient | Aripiprazole |
| Dosage Form | Tablet, orally disintegrating; Tablet |
| Route | orally disintegrating; oral |
| Strength | 5mg; 2mg; 30mg; 10mg; 15mg; 20mg |
| Market Status | Tentative Approval |
| Company | Apotex; Alembic Pharms; Torrent Pharms; Zydus Pharms Usa; Barr Labs; Sun Pharma Global |
| 3 of 4 | |
|---|---|
| Drug Name | Abilify |
| PubMed Health | Aripiprazole (Injection) |
| Drug Classes | Antipsychotic |
| Drug Label | Aripiprazole is a psychotropic drug that is available as ABILIFY (aripiprazole) Tablets, ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets, ABILIFY (aripiprazole) Oral Solution, and ABILIFY (aripiprazole) Injection, a solution for... |
| Active Ingredient | Aripiprazole |
| Dosage Form | Solution; Injectable; Tablet, orally disintegrating; Tablet |
| Route | Intramuscular; Oral |
| Strength | 30mg; 10mg; 5mg; 9.75mg/1.3ml (7.5mg/ml); 15mg; 1mg/ml; 20mg; 2mg |
| Market Status | Prescription |
| Company | Otsuka |
| 4 of 4 | |
|---|---|
| Drug Name | Aripiprazole |
| PubMed Health | Aripiprazole |
| Drug Classes | Antipsychotic |
| Drug Label | Aripiprazole is a psychotropic drug that is available as ABILIFY (aripiprazole) Tablets, ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets, ABILIFY (aripiprazole) Oral Solution, and ABILIFY (aripiprazole) Injection, a solution for... |
| Active Ingredient | Aripiprazole |
| Dosage Form | Tablet, orally disintegrating; Tablet |
| Route | orally disintegrating; oral |
| Strength | 5mg; 2mg; 30mg; 10mg; 15mg; 20mg |
| Market Status | Tentative Approval |
| Company | Apotex; Alembic Pharms; Torrent Pharms; Zydus Pharms Usa; Barr Labs; Sun Pharma Global |
Antipsychotic Agents
National Library of Medicine's Medical Subject Headings. Aripiprazole. Online file (MeSH, 2014). Available from, as of December 18, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Aripiprazole is used IM for the acute management of agitation associated with schizophrenia or bipolar disorder, mixed or manic, in adults for whom treatment with aripiprazole is appropriate and who require an IM antipsychotic agent for rapid control of behaviors that interfere with diagnosis and care (e.g., threatening behaviors, escalating or urgently distressing behavior, self-exhausting behavior).
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2428
Aripiprazole is used orally for the acute treatment of irritability associated with autistic disorder.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2428
Aripiprazole is used orally as an adjunct to antidepressants for the acute treatment of major depressive disorder in adults.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2428
For more Therapeutic Uses (Complete) data for ARIPIPRAZOLE (6 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis. /Included in label/
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of March 31, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
/BOXED WARNING/ WARNING: INCREASED SUICIDALTHOUGHTS AND BEHAVIORS. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of adjunctive ABILIFY or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. ABILIFY is not approved for use in pediatric patients with depression. /Included in label/
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of March 31, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
Contraindications: Known hypersensitivity reaction to aripiprazole or any ingredient in the formulation; such reactions have ranged from pruritus/urticaria to anaphylaxis.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2429
Safety and effectiveness in pediatric patients with bipolar mania were established in a 4-week, placebo-controlled clinical trial in 197 pediatric patients aged 10 to 17 years. The incidence of discontinuation due to adverse reactions between aripiprazole-treated and placebo-treated pediatric patients (10 to 17 years) was 7% and 2%, respectively. Commonly observed adverse reactions associated with the use of aripiprazole in pediatric patients with bipolar mania (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) somnolence, extrapyramidal disorder, fatigue, nausea, akathisia, blurred vision, salivary hypersecretion, and dizziness. Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of September 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
For more Drug Warnings (Complete) data for ARIPIPRAZOLE (27 total), please visit the HSDB record page.
Aripiprazole is indicated for manic and mixed episodes associated with bipolar I disorder, irritability associated with autism spectrum disorder, treatment of schizophrenia, treatment of Tourette's disorder, and as an adjunctive treatment of major depressive disorder. An injectable formulation of aripiprazole is indicated for agitation associated with schizophrenia or bipolar mania.
FDA Label
Aripiprazole Accord is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Aripiprazole Accord is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Aripiprazole Accord is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Aripiprazole Mylan Pharma is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Aripiprazole Mylan Pharma is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Aripiprazole Mylan Pharma is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Abilify is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Abilify is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Abilify is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Aripiprazole Zentiva is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Aripiprazole Zentiva is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Aripiprazole Zentiva is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Aripiprazole Sandoz is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Aripiprazole Sandoz is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Aripiprazole Sandoz is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Maintenance treatment of schizophrenia in adult patients stabilised with oral aripiprazole.
Treatment of bipolar affective disorder, Treatment of schizophrenia
Aripiprazole has high affinity for serotonin type 2 (5HT2), dopamine type 2 (D2), alpha1 and 2 adrenergic, and H1 histaminergic receptors. It also acts on a number of other receptors with lower affinity. The exact method by which aripiprazole's action on these receptors translates to a clinically relevant effect is not yet known.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
Dopamine D2 Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of DOPAMINE D2 RECEPTORS. (See all compounds classified as Dopamine D2 Receptor Antagonists.)
Serotonin 5-HT1 Receptor Agonists
Endogenous compounds and drugs that specifically stimulate SEROTONIN 5-HT1 RECEPTORS. Included under this heading are agonists for one or more of the specific 5-HT1 receptor subtypes. (See all compounds classified as Serotonin 5-HT1 Receptor Agonists.)
Serotonin 5-HT2 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT2 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT2 RECEPTOR AGONISTS. Included under this heading are antagonists for one or more specific 5-HT2 receptor subtypes. (See all compounds classified as Serotonin 5-HT2 Receptor Antagonists.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
N05AX12
N05AX12
N05AX12
N05AX12
N05AX12
N05AX12
N05AX12
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AX - Other antipsychotics
N05AX12 - Aripiprazole
Absorption
Aripiprazole tablets are 87% bioavailable and reach peak plasma concentrations in 3 to 5 hours. These tablets can be taken with or without food, but a high fat meal can delay the time to max concentration by 3 hours and up to 12 hours for the active metabolite.
Route of Elimination
25% of a given dose will be eliminated in urine and 55% in the feces. <1% of a dose is eliminated in the urine as unmetabolized aripiprazole and approximately 18% of a dose will be eliminated in the feces unmetabolized.
Volume of Distribution
404L or 4.9L/kg.
Clearance
0.8mL/min/kg. Other studies have reported a clearance rate of 32971042mL/hr.
Oral availability 87%. Aripiprazole is well absorbed and can be administered with or without food. Administration with a high fat meal did not affect the Cmax or AUC, but delayed Tmax by 3 hours for aripiprazole, and 12 hours for dehydro-aripiprazole.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2431
Time to peak concentration: Peak plasma concentrations: within 3 to 5 hours.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2431
The steady-state volume of distribution of aripiprazole following intravenous administration is high (404 L or 4.9 L/kg), indicating extensive extravascular distribution. At therapeutic concentrations, aripiprazole and its major metabolite are greater than 99% bound to serum proteins, primarily to albumin.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2431
There was dose-dependent D2-receptor occupancy indicating brain penetration of aripiprazole in healthy human volunteers administered 0.5 to 30 mg per day.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2431
For more Absorption, Distribution and Excretion (Complete) data for ARIPIPRAZOLE (8 total), please visit the HSDB record page.
Metabolism of aripiprazole is predominantly hepatic, mediated mostly by cytochrome P450 (CYP)3A4 and CYP2D6. These enzymes perform dehydrogenation and hydroxylation while CYP3A4 alone performs N-dealkylation. At any given time, the active metabolite dehydro-aripiprazole is approximately 40% of the drug available in plasma.
Aripiprazole is extensively metabolized in the liver principally via dehydrogenation, hydroxylations, and N-dealkylation by the cytochrome P-450 (CYP) 2D6 and 3A4 isoenzymes. The major active metabolite of aripiprazole, dehydro-aripiprazole, exhibits affinity for D2 receptors similar to that of the parent compound and represents approximately 40% of the aripiprazole area under the concentration-time curve (AUC) in plasma. Steady-state plasma concentrations of both aripiprazole and dehydro-aripiprazole are achieved within 14 days.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2433
ABILIFY activity is presumably primarily due to the parent drug, aripiprazole, and to a lesser extent, to its major metabolite, dehydro-aripiprazole, which has been shown to have affinities for D2 receptors similar to the parent drug and represents 40% of the parent drug exposure in plasma.
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of September 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
Aripiprazole has known human metabolites that include 2,3-dichlorophenylpiperazine, 4-Hydroxyaripiprazole, 4-[(2-oxo-3,4-dihydro-1H-quinolin-7-yl)oxy]butanal, and dehydro-aripiprazole.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half life of aripiprazole is 75 hours while the half life of the active metabolite is 94 hours. For populations that are poor CYP2D6 metabolizers, the half life of aripiprazole is 146 hours and these patients should be treated with half the normal dose. Other studies have reported a half life of 61.0319.59 hours for aripiprazole and 279299 hours for the active metabolite.
The mean elimination half-lives are about 75 hours and 94 hours for aripiprazole and dehydro-aripiprazole, respectively.
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of September 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
The antipsychotic action of aripiprazole is likely due to the agonism of D2 and 5-HT1A receptors though the exact mechanism has not been defined. Some adverse effects may be due to action on other receptors. For example, orthostatic hypotension may be explained by antagonism of the adrenergic alpha1 receptors.
The exact mechanism of antipsychotic action of aripiprazole has not been fully elucidated but, like that of other atypical antipsychotic agents (e.g.,olanzipine, risperidone, ziprasidon), may involve the drug's activity at dopamine D2 and serotonin type (5-HT1A) and type 2 (5-HT2A) receptors. However, aripiprazole appears to differ from other atypical antipsychotic agents because the drug demonstrates partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. Antagonism at other receptors (e.g., alpha1-adrenergic receptors, histamine H1 receptors) may contribute to other therapeutic and adverse effects (e.g., orthostatic hypotension, somnolence) observed with aripiprazole.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2432
...Aripiprazole exhibits typical antagonism at dopamine (D2) receptors in the mesolimbic pathway, as well as having unique partial agonist activity at D2 receptors in the mesocortical pathway. As exemplified by other atypical antipsychotics, it displays strong 5-HT(2a) receptor antagonism and is similar to ziprasidone in also having agonistic activity at the 5-HT(1a) receptor. Among the atypical antipsychotics, aripiprazole displays the lowest affinity for alpha(1)adrenergic (alpha(1)), histamine (H1) and muscarinic (M1) receptors. This combination of effects may be responsible for its efficacy in positive and negative symptoms of schizophrenia and in bipolar disorder. ...Other early data suggest that aripiprazole may induce reductions in plasma prolactin, as well as in plasma glucose and lipid profiles ...
PMID:12472374 Goodnick PJ, Jerry JM; Expert Opin Pharmacother 3 (12): 1773-81 (2002)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
15
PharmaCompass offers a list of Aripiprazole API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aripiprazole manufacturer or Aripiprazole supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aripiprazole manufacturer or Aripiprazole supplier.
PharmaCompass also assists you with knowing the Aripiprazole API Price utilized in the formulation of products. Aripiprazole API Price is not always fixed or binding as the Aripiprazole Price is obtained through a variety of data sources. The Aripiprazole Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aripiprazole manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aripiprazole, including repackagers and relabelers. The FDA regulates Aripiprazole manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aripiprazole API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aripiprazole manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aripiprazole supplier is an individual or a company that provides Aripiprazole active pharmaceutical ingredient (API) or Aripiprazole finished formulations upon request. The Aripiprazole suppliers may include Aripiprazole API manufacturers, exporters, distributors and traders.
click here to find a list of Aripiprazole suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Aripiprazole DMF (Drug Master File) is a document detailing the whole manufacturing process of Aripiprazole active pharmaceutical ingredient (API) in detail. Different forms of Aripiprazole DMFs exist exist since differing nations have different regulations, such as Aripiprazole USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Aripiprazole DMF submitted to regulatory agencies in the US is known as a USDMF. Aripiprazole USDMF includes data on Aripiprazole's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Aripiprazole USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Aripiprazole suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Aripiprazole Drug Master File in Japan (Aripiprazole JDMF) empowers Aripiprazole API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Aripiprazole JDMF during the approval evaluation for pharmaceutical products. At the time of Aripiprazole JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Aripiprazole suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Aripiprazole Drug Master File in Korea (Aripiprazole KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Aripiprazole. The MFDS reviews the Aripiprazole KDMF as part of the drug registration process and uses the information provided in the Aripiprazole KDMF to evaluate the safety and efficacy of the drug.
After submitting a Aripiprazole KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Aripiprazole API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Aripiprazole suppliers with KDMF on PharmaCompass.
A Aripiprazole CEP of the European Pharmacopoeia monograph is often referred to as a Aripiprazole Certificate of Suitability (COS). The purpose of a Aripiprazole CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Aripiprazole EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Aripiprazole to their clients by showing that a Aripiprazole CEP has been issued for it. The manufacturer submits a Aripiprazole CEP (COS) as part of the market authorization procedure, and it takes on the role of a Aripiprazole CEP holder for the record. Additionally, the data presented in the Aripiprazole CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Aripiprazole DMF.
A Aripiprazole CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Aripiprazole CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Aripiprazole suppliers with CEP (COS) on PharmaCompass.
A Aripiprazole written confirmation (Aripiprazole WC) is an official document issued by a regulatory agency to a Aripiprazole manufacturer, verifying that the manufacturing facility of a Aripiprazole active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Aripiprazole APIs or Aripiprazole finished pharmaceutical products to another nation, regulatory agencies frequently require a Aripiprazole WC (written confirmation) as part of the regulatory process.
click here to find a list of Aripiprazole suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Aripiprazole as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Aripiprazole API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Aripiprazole as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Aripiprazole and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Aripiprazole NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Aripiprazole suppliers with NDC on PharmaCompass.
Aripiprazole Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aripiprazole GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aripiprazole GMP manufacturer or Aripiprazole GMP API supplier for your needs.
A Aripiprazole CoA (Certificate of Analysis) is a formal document that attests to Aripiprazole's compliance with Aripiprazole specifications and serves as a tool for batch-level quality control.
Aripiprazole CoA mostly includes findings from lab analyses of a specific batch. For each Aripiprazole CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aripiprazole may be tested according to a variety of international standards, such as European Pharmacopoeia (Aripiprazole EP), Aripiprazole JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aripiprazole USP).

