A review of the Drug Master Files (DMFs) submitted to the United States Food and Drug Administration (FDA) from October 2016 to March 2017 (the fourth quarter of 2016 and the first quarter of 2017) indicates an extremely robust pharmaceutical industry in India. However, the filing make one question an article we had carried earlier this month on the end of India’s pharma honeymoon.
India filed more than half the DMFs submitted
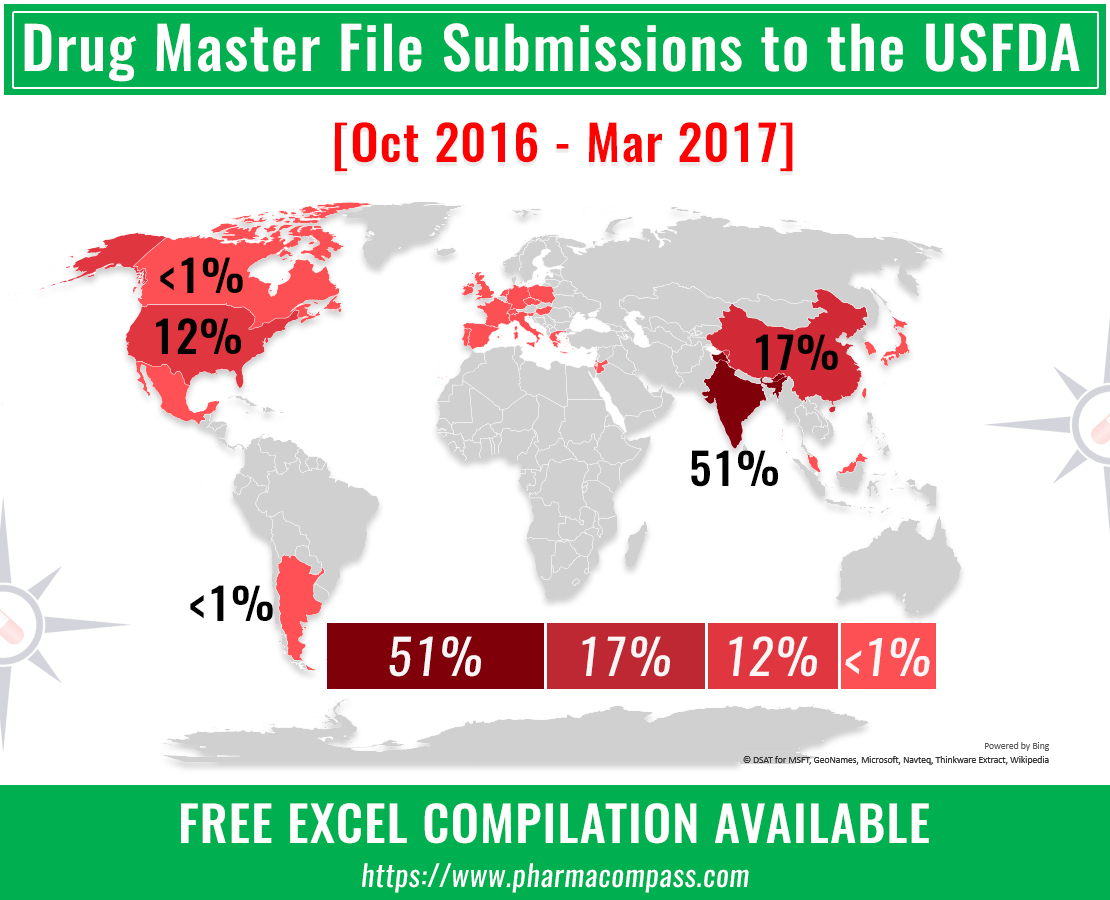
Indian companies filed more than half (176) of the 345 DMFs submitted with the FDA. China (60) came a distant second, followed by the United States (40). While DMF submissions were made from 26 other countries, the activity levels seen were a far cry from what was seen in India and China.
In our past compilation for the second and third quarters of 2016, Macleods Pharmaceuticals (14 DMFs) had pipped MSN Laboratories (13 DMFs) to become the leading filer from India.
However, in the recent review period, MSN bounced back with 41 DMF submissions, compared to six for Macleod. Leading Indian pharmaceutical majors such as Aurobindo, Sun Pharma, Amneal, Mylan’s India operations, Hetero, Cipla and Jubilant had six or more filings each.
Ajinomoto’s North American operations filed 11 DMFs for various amino acids and led the submissions for products manufactured in the United States.
Tianjin Weijie Pharmaceutical led the pack from China, with eight DMF submissions.
Over the review period, a total of 345 submissions were made to the FDA, almost similar to the 379 DMFs filed during the second and third quarters of 2016.
The next FTF challenges
Since APIs form the building blocks of finished formulations, DMF submissions give a sneak preview into the next possible first-to-file (FTF) generic challenges to patented drugs.
In December 2016, Indian drug major Sun Pharmaceutical Industries announced it will acquire a branded oncology product, Odomzo, from Novartis for an upfront payment of US$ 175 million.
A little over three months after the announcement, MSN Labs filed the first DMF for Odomzo’s active pharmaceutical ingredient (API), sonidegib phosphate. Odomzo was approved by the FDA in July 2015 and is indicated for the treatment of adult patients with locally advanced basal cell carcinoma.
While patents for Novartis’ drugs Tafinlar and Mekinist are not scheduled to expire until 2029, Novartis should prepare for a generic competitor as DMFs were filed for the APIs used in both drugs by MSN Labs. The drugs had combined sales in 2016 of US$ 672 million.
In addition, MSN Labs also filed the first DMF for nintedanib esylate, the API used in Boehringer Ingelheim’s idiopathic pulmonary fibrosis treatment, Ofev, and for cabozatinib (S)-malate, the API used by Exelixis for its kidney cancer treatment — Cabometyx.
While in most cases MSN’s DMF is the first one to get filed, in the case of Helsinn’s Akynzeo, which is used to prevent chemotherapy-induced nausea and vomiting, Apicore US also filed a DMF along with MSN.
Although sales forecasts for Bayer’s pulmonary arterial hypertension treatment — Adempas (riociguat) — were lowered as the drug did not get the expected sales start and planned label expansions did not materialize, this did not stop MSN Labs from filing the first DMF for this product as well.
Another drug which is struggling to meet analyst expectations is Novartis’ Entresto. Once considered “one of the most important products in the company's history” with an expectation to reach US$ 10 billion in peak sales, the drug generated sales of only US$ 170 million in 2016. Regardless, Mylan filed the first DMF for the API.
Another drug where MSN Labs did not file the first DMF was for AstraZeneca’s ovarian cancer treatment, Lynparza (olaparib). Alp Pharm Beijing submitted the DMF for the drug which generated US$ 218 million in sales in 2016 and a figure Bernstein Research analyst Timothy Anderson forecasts will grow to US$ 684 million by 2020.
The onslaught on Novartis’ portfolio is not limited to only patented products as Lamprene, a product which has been on the market since 1986, and not had any generic competitor, had Zhejiang Huahai file a DMF indicating a generic competitor maybe on the horizon soon.
Mylan’s Sotradecol has been the only injectable form of sodium tetradecyl sulfate on the market since 2004. That monopoly may end soon as the FDA completed the review of a DMF filed for the API early this year.
Most actively filed products — vortioxetine hydrobromide and dolutegravir sodium
The most actively filed DMFs, with seven filings each, were those for Takeda’s depression drug Trintellix (vortioxetine hydrobromide) and GSK’s HIV treatment dolutegravir sodium.
Johnson & Johnson’s diabetes treatment Invokana (canagliflozin), which recently demonstrated that it decreased the risk of heart attacks and strokes, while increasing the risk of amputation, particularly of toes, had six new DMFs filed.
There were also six new DMFs filed for apremilast, five for darunavir and four each for dapagliflozin and rivaroxaban.
Filings for new drugs under development
Helsinn is moving ahead with the filing of anamorelin hydrochloride, as it filed DMFs for the API and the 100 mg tablets, a product which was welcomed enthusiastically by oncology experts as it raised hopes to be a drug for cancer cachexia, the extreme wasting seen at the end stages of the disease.
But those hopes were recently dashed, as a review of the clinical data by the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) found only "marginal" effects and recommended that the product be refused marketing authorization in Europe.
Now it remains to be seen what the FDA’s verdict on this drug will be.
AB Science’s Masitinib has been in the news recently as an EMA committee announced the drug, developed for a range of cancers, could not be approved due to “serious failings” in the way clinical studies were conducted.
However, this did not stop Excella GmbH from filing its second DMF for the API.
Multiple sclerosis treatment dimethyl fumarate (Biogen’s Tecfidera) generated sales of US$ 3.97 billion in 2016 and is projected to achieve US$ 5.56 billion by 2020.
While there are now 28 DMFs filed for dimethyl fumarate, in March this year Alkermes announced the initiation of a new phase 3 study of ALKS 8700, a novel, oral monomethyl fumarate (MMF) prodrug candidate in development for the treatment of relapsing forms of multiple sclerosis.
It remains to be seen when Alkermes’ product will get approved. However, MSN Labs followed Honour Labs to file the second DMF for this product.
A new submission for deslorelin acetate (an injectable gonadotropin releasing hormone super-agonist) indicates there maybe a new drug development underway for this age-old peptide as currently there are no approved drugs in the US.
A similar situation seems to exist for taurolidine, an antimicrobial that seeks to prevent infections in catheters.
Vasudha Pharma’s filing of cisapride monohydrate comes as a surprise. The product, which was launched by Janssen for increased motility of the gastrointestinal tract, was later withdrawn from the US market due to concerns of fatalities linked to cardiac arrhythmias.
The product, however, continues to be exported from India to countries like Switzerland, Thailand, Mexico, China and Canada.
Our view
The last quarter of 2016 and the first quarter of 2017 clearly demonstrate an API industry in India and China, which is extremely active with new product development, regardless of disappointing financial results posted by major pharma companies and growing concerns over regulatory non-compliances.
Given the market headwinds and increased compliance expectations, it remains to be seen how many of these DMFs filed actually result in drugs reaching the market.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Drug Master File Submissions to the USFDA by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”




