By PharmaCompass
2025-08-07
Impressions: 6700
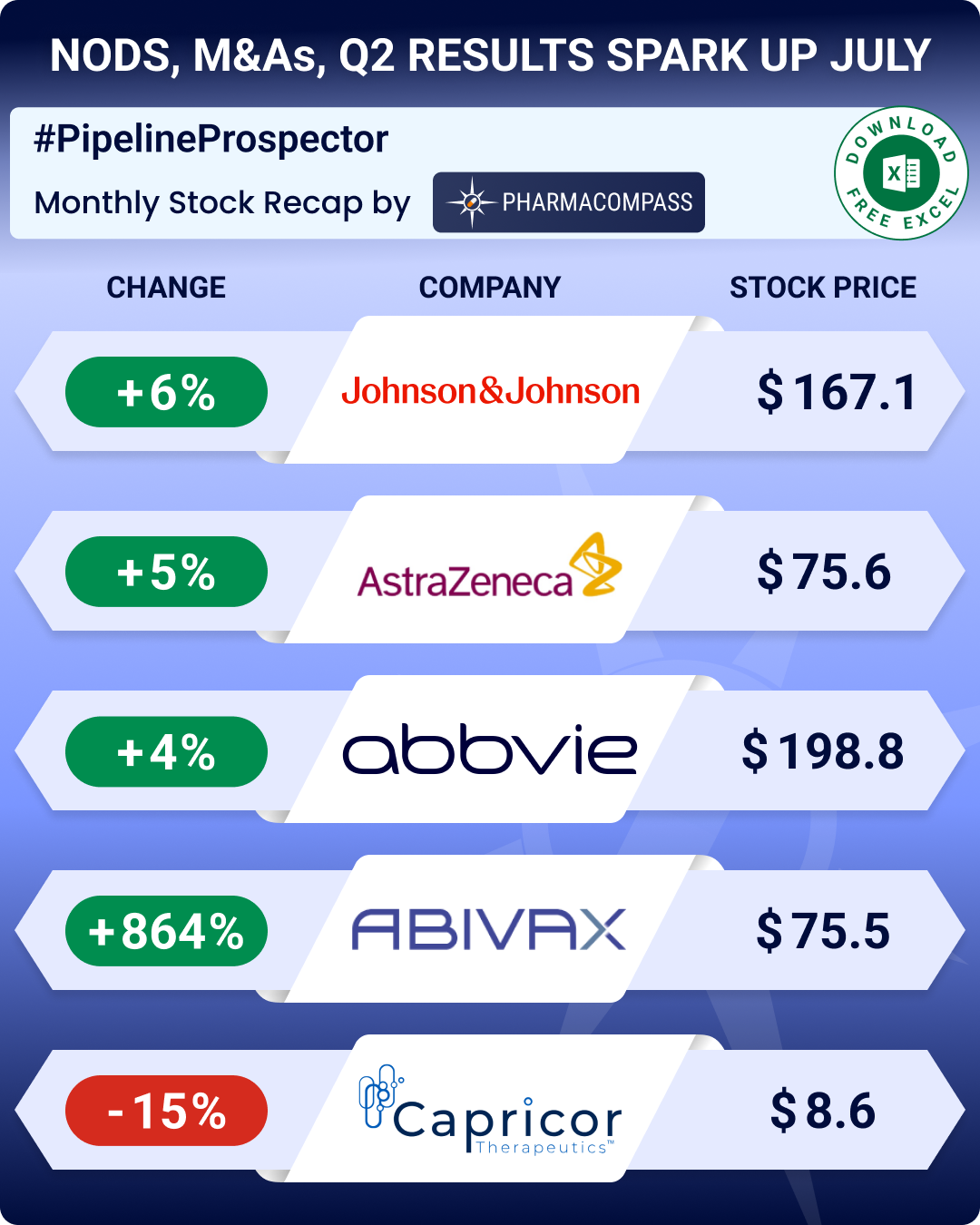
In July, the pharmaceutical industry witnessed several deals and mergers and acquisitions (M&As). Big pharmaceutical companies such as AbbVie, Johnson & Johnson and Novo Nordisk posted robust second quarter (Q2) results. AbbVie and J&J also raised their outlook for the full year 2025.
In the face of looming import tariffs on pharmaceuticals, companies continued to announce significant investments in the US. While AstraZeneca announced an investment of US$ 50 billion to expand manufacturing and research capabilities in America by 2030, Biogen announced an additional investment of US$ 2 billion at its existing manufacturing plants in North Carolina.
These developments appeared to have pushed the pharma indices upwards. The Nasdaq Biotechnology Index (NBI) gained 5.74 percent from 4,219.14 to 4,461.23, while the SPDR S&P Biotech ETF (XBI) rose 4.58 percent from 82.46 to 86.24, and the S&P Biotechnology Select Industry Index (SPSIBI) surged 3.29 percent from 6,459.24 to 6,671.56.
Access the Pipeline Prospector Dashboard for July 2025 Newsmakers (Free Excel)
Merck to acquire London-based Verona for US$ 10 bn, GSK buys Hengrui for US$ 12 bn
Amongst the notable M&As was American pharmaceutical giant Merck’s acquisition of London‑based Verona Pharma for approximately US$ 10 billion. With this acquisition, Merck will get access to Verona’s Ohtuvayre (ensifentrine), an FDA-approved treatment for chronic obstructive pulmonary disease (COPD) that generated US$ 71.3 million in revenues during Q1 2025. This acquisition will help Merck diversify ahead of the 2028 patent expiration of its cancer blockbuster, Keytruda (pembrolizumab).
Similarly, GSK signed an up to US$ 12 billion biobucks deal with China’s Hengrui Pharma to work on up to 12 drugs. GSK is paying Hengrui US$ 500 million upfront. The programs were selected to complement GSK’s extensive respiratory, immunology, inflammation and oncology pipelines.
Sanofi announced the acquisition of London-based biotech Vicebio for an upfront payment of US$ 1.15 billion in order to expand its respiratory vaccine portfolio. And vaccine maker Bavarian Nordic announced that a consortium led by Nordic Capital and Permira has made an offer of around US$ 3 billion to acquire it.
In deals, Roche‑backed Chugai Pharmaceutical and Singapore‑based AI‑driven biotech Gero have launched a joint research and licensing agreement targeting age‑related diseases in a deal valued at up to US$ 1 billion. And Argenx entered into a multi-target research collaboration with Unnatural Products (UNP), a California-based biotech firm specializing in AI-driven macrocyclic peptide therapeutics.
Access the Pipeline Prospector Dashboard for July 2025 Newsmakers (Free Excel)
FDA approves Regeneron’s blood cancer med, okays PTC’s drug to treat genetic disorder
Last month, the US Food and Drug Administration (FDA) granted an accelerated approval to Regeneron’s Lynozyfic (linvoseltamab-gcpt), a monoclonal antibody that treats adult patients with relapsed or refractory (R/R) multiple myeloma who have received at least four prior lines of therapy. Multiple myeloma is a cancer that forms in a type of white blood cell, known as plasma.
The agency also granted accelerated approval to Zegfrovy (sunvozertinib), developed by Dizal Pharmaceutical, for the treatment of adults with locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring EGFR exon 20 insertion mutations, making it the only drug for this condition. This approval is specifically for patients whose disease has progressed on or after platinum-based chemotherapy.
The agency also approved PTC Therapeutics’ oral drug — Sephience (sepiapterin) — to treat a rare genetic disorder known as phenylketonuria (PKU). In PKU, the body can’t properly break down an amino acid known as phenylalanine, leading to its buildup in the blood which can potentially damage the brain.
KalVista Pharmaceuticals secured FDA approval for Ekterly (sebetralstat), marking the first-ever oral, on‑demand treatment for hereditary angioedema (HAE), a rare and potentially fatal swelling disorder.
Access the Pipeline Prospector Dashboard for July 2025 Newsmakers (Free Excel)
Astra’s baxdrostat lowers BP in phase 3 trial; FDA declines to approve Ultragenyx’s gene therapy
AstraZeneca’s experimental drug baxdrostat significantly lowered blood pressure in a phase 3 trial on patients with treatment-resistant hypertension. AstraZeneca had acquired baxdrostat through its 2023 purchase of CinCor Pharma for US$ 1.8 billion. The company expects peak annual sales of the drug to exceed US$ 5 billion.
Clinical stage biotech Abivax presented top-line data from the twin phase 3 trials of its lead ulcerative colitis candidate — obefazimod. The drug produced a statistically significant pooled remission rate of 16.4 percent across both trials, largely hitting their primary endpoints.
There was considerable negative news from clinical trials, including the death of a 51-year-old man participating in Sarepta’s phase 1 study for limb-girdle muscular dystrophy. This person was taking another gene therapy — Sarepta’s SRP-9004. The previous two deaths were of teenaged patients taking its gene therapy Elevidys. Post this news, Sarepta has decided to let go of 500 employees (36 percent of its workforce) and is also halting the development of several gene therapies for a group of muscle wasting disorders.
The agency also raised efficacy concerns over the use of Otsuka Pharma’s drug — Rexulti (brexpiprazole) — in combination with Viatris’ antidepressant Zoloft (sertraline) for the treatment of adults with post-traumatic stress disorder (PTSD). FDA has cited inconsistent trial results and a modest treatment effect and is insisting on another study on Rexulti.
FDA declined to approve UX111, Ultragenyx’s gene therapy for Sanflilippo syndrome type A, a rare disease that causes progressive damage to the central nervous system. In the complete response letter (CRL), the agency has requested for additional information related to the company’s production processes and facilities.
The FDA also declined to approve Capricor Therapeutics’ Deramiocel (CAP-1002) , a lead cell therapy candidate for the treatment of cardiomyopathy associated with Duchenne muscular dystrophy (DMD). In its CRL, FDA said the evidence submitted for the therapy does not meet efficacy requirements and has asked for more data.
Access the Pipeline Prospector Dashboard for July 2025 Newsmakers (Free Excel)
Our view
Although the pharma indices are looking up and we are witnessing substantial M&A activity, we know that the drug industry is under considerable pressure from US President Donald Trump’s tariffs and other policies. His administration has sent letters to 17 major drug companies giving them 60 days to cut prices for US consumers to the lowest prices paid by other countries.
The new tariffs, be it via the Europe-US trade deal or the increased tariffs on Indian goods, are likely to hurt the Americal healthcare system and raise costs. Moreover, Trump has said tariffs on drugs imported into the US could reach up to 250 percent in another 12 to 18 months. Once costs begin to escalate, the ripple effects across healthcare systems could be profound.
Access the Pipeline Prospector Dashboard for July 2025 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in July 2025
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : APPROVALS, M&AS, Q2 RESULTS SPARK up July by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







