
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Weekly News Recap #Phispers
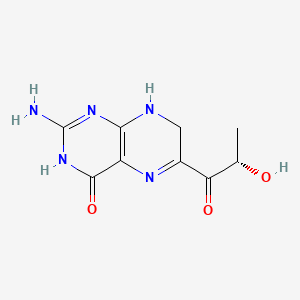

1. 2-amino-6-(s)-lactoyl-7,8-dihydro-4(3h)- Pteridinone
2. 2-amino-7,8-dihydro-6-((2s)-2-hydroxy-1-oxopropyl)-4(3h)-pteridinone Acid
3. 4(3h)-pteridinone, 2-amino-7,8-dihydro-6-((2s)-2-hydroxy-1-oxopropyl)-
4. L-sepiapterin
5. Sepia-pterin
6. Sepiapterin-c
7. Sepiapterine
1. L-sepiapterin
2. 17094-01-8
3. Sepiapterine
4. Sepiapterin [usan]
5. Lopac-s-154
6. Cjq26ko7hp
7. (s)-2-amino-6-(2-hydroxypropanoyl)-7,8-dihydropteridin-4(3h)-one
8. Cnsa-001
9. Ptc923
10. 2-amino-6-(s)-lactoyl-7,8-dihydro-4(3h)-pteridinone
11. 2-amino-6-[(2s)-2-hydroxypropanoyl]-7,8-dihydro-3h-pteridin-4-one
12. 4(3h)-pteridinone, 2-amino-7,8-dihydro-6-((2s)-2-hydroxy-1-oxopropyl)-
13. Unii-cjq26ko7hp
14. 4(3h)-pteridinone, 2-amino-7,8-dihydro-6-[(2s)-2-hydroxy-1-oxopropyl]-
15. 4(3h)-pteridinone,2-amino-7,8-dihydro-6-[(2s)-2-hydroxy-1-oxopropyl]-
16. Sepiapterin, Solid
17. Sepiapterin [inn]
18. Lopac0_001123
19. 6-lactoyl-7,8-dihydropterin
20. Mls002153268
21. Schembl258399
22. Chembl1255653
23. Dtxsid40937902
24. Ptc-923
25. Hms2234o10
26. Hms3263b07
27. Tox21_501123
28. 2-amino-6-[(2s)-2-hydroxypropanoyl]-7,8-dihydro-1h-pteridin-4-one
29. Who 11848
30. Zinc17721961
31. Akos022181294
32. Ccg-205198
33. Lp01123
34. Sdccgsbi-0051091.p002
35. Ncgc00015913-01
36. Ncgc00094391-01
37. Ncgc00094391-02
38. Ncgc00094391-03
39. Ncgc00094391-05
40. Ncgc00261808-01
41. Smr001230707
42. Hy-112234
43. Cs-0044215
44. Eu-0101123
45. S-154
46. C00835
47. Sr-01000075522
48. Q2271580
49. Sr-01000075522-1
50. (s)-2-amino-6-(2-hydroxypropanoyl)-7,8-dihydropteridin-4(1h)-one
51. (s)-2-amino-7,8-dihydro-6-(2-hydroxy-1-oxopropyl)-4(1h)-pteridinone
52. 1-(2-amino-7,8-dihydro-4-hydroxy-6-pteridinyl)-2-hydroxy-1-propanone
53. 2-amino-6-[(2s)-2-hydroxypropanoyl]-1,4,7,8-tetrahydropteridin-4-one
54. 2-amino-7,8-dihydro-6-[(2s)-2-hydroxy-1-oxopropyl]-4(1h)pteridinone
55. S(-)-2-amino-7,8-dihydro-6-(2-hydroxy-1-oxopropyl)-4(1h)-pteridione
56. 2-amino-7,8-dihydro-6-((2s)-2-hydroxy-1-oxopropyl)-4(3h)-pteridinone
57. 4(1h)-pteridinone, 2-amino-7,8-dihydro-6-(2-hydroxy-1-oxopropyl)-, (s)-
| Molecular Weight | 237.22 g/mol |
|---|---|
| Molecular Formula | C9H11N5O3 |
| XLogP3 | -2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 237.08618923 g/mol |
| Monoisotopic Mass | 237.08618923 g/mol |
| Topological Polar Surface Area | 129 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 491 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sephience (sepiapterin) is a PAH activator indicated for the treatment of hyperphenylalaninemia in adult and pediatric patients 1 month of age and older with PKU.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Sephience
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 28, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
US FDA approves PTC Therapeutics' metabolic disorder drug
Details : Sephience (sepiapterin) is a PAH activator indicated for the treatment of hyperphenylalaninemia in adult and pediatric patients 1 month of age and older with PKU.
Product Name : Sephience
Product Type : Miscellaneous
Upfront Cash : Inapplicable
July 28, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sephience (sepiapterin) is an oral formulation of sepiapterin, has a dual mechanism of action to increase activity of the phenylalanine hydroxylase enzyme. It is being evaluated for Phenylketonuria.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Sephience
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 25, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
PTC gets Positive CHMP Opinion for Sephience in Treating PKU in Children and Adults
Details : Sephience (sepiapterin) is an oral formulation of sepiapterin, has a dual mechanism of action to increase activity of the phenylalanine hydroxylase enzyme. It is being evaluated for Phenylketonuria.
Product Name : Sephience
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 25, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PTC923 (sepiapterin) is an oral formulation, that has a dual moa to increase the activity of the PAH enzyme. It is being evaluated for the treatment of pediatric and adult phenylketonuria patients.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 20, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
PTC Therapeutics Presents New Sepiapterin Data from Ongoing Studies
Details : PTC923 (sepiapterin) is an oral formulation, that has a dual moa to increase the activity of the PAH enzyme. It is being evaluated for the treatment of pediatric and adult phenylketonuria patients.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 20, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PTC923 (sepiapterin) is an oral formulation, that has a dual moa to increase the activity of the PAH enzyme. It is being evaluated for the treatment of pediatric and adult phenylketonuria patients.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
PTC’s Sepiapterin NDA Accepted by FDA for Phenylketonuria
Details : PTC923 (sepiapterin) is an oral formulation, that has a dual moa to increase the activity of the PAH enzyme. It is being evaluated for the treatment of pediatric and adult phenylketonuria patients.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PTC923 (sepiapterin) is a precursor to intracellular tetrahydrobiopterin that acts as nitric oxide synthase modulator. It is being evaluated for the treatment of Phenylketonuria.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
PTC Announces Submission of Sepiapterin MAA for Treatment of PKU to EMA
Details : PTC923 (sepiapterin) is a precursor to intracellular tetrahydrobiopterin that acts as nitric oxide synthase modulator. It is being evaluated for the treatment of Phenylketonuria.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 28, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Sepiapterin is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Phenylketonurias.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Study of Sepiapterin in Participants With Phenylketonuria (PKU)
Details : Sepiapterin is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Phenylketonurias.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 08, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PTC923 (sepiapterin) is a precursor to intracellular tetrahydrobiopterin, which is a critical enzymatic cofactor involved in the metabolism and synthesis of numerous metabolic products and has the potential to treat the broad range of PKU patients.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : PTC923 (sepiapterin) is a precursor to intracellular tetrahydrobiopterin, which is a critical enzymatic cofactor involved in the metabolism and synthesis of numerous metabolic products and has the potential to treat the broad range of PKU patients.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PTC923 is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Phenylketonurias.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 21, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Long-Term Safety Study of PTC923 in Participants With Phenylketonuria
Details : PTC923 is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Phenylketonurias.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 21, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PTC923 is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Phenylketonurias.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Genetic Disease Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 29, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Genetic Disease
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Study of PTC923 in Participants With Phenylketonuria
Details : PTC923 is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Phenylketonurias.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 29, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Censa Pharmaceuticals acquisition diversifies and strengthens PTC 's portfolio of rare disorders by adding the lead orphan metabolic disease drug, CNSA-001.
Lead Product(s): Sepiapterin,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: PTC Therapeutics
Deal Size: $366.0 million Upfront Cash: $10.0 million
Deal Type: Acquisition May 06, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Sepiapterin,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : PTC Therapeutics
Deal Size : $366.0 million
Deal Type : Acquisition
PTC Therapeutics to Acquire Censa Pharmaceuticals
Details : Censa Pharmaceuticals acquisition diversifies and strengthens PTC 's portfolio of rare disorders by adding the lead orphan metabolic disease drug, CNSA-001.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : $10.0 million
May 06, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patents & EXCLUSIVITIES
ABOUT THIS PAGE
46
PharmaCompass offers a list of Sepiapterin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sepiapterin manufacturer or Sepiapterin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sepiapterin manufacturer or Sepiapterin supplier.
PharmaCompass also assists you with knowing the Sepiapterin API Price utilized in the formulation of products. Sepiapterin API Price is not always fixed or binding as the Sepiapterin Price is obtained through a variety of data sources. The Sepiapterin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Sepiapterin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Sepiapterin, including repackagers and relabelers. The FDA regulates Sepiapterin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Sepiapterin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Sepiapterin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Sepiapterin supplier is an individual or a company that provides Sepiapterin active pharmaceutical ingredient (API) or Sepiapterin finished formulations upon request. The Sepiapterin suppliers may include Sepiapterin API manufacturers, exporters, distributors and traders.
click here to find a list of Sepiapterin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Sepiapterin written confirmation (Sepiapterin WC) is an official document issued by a regulatory agency to a Sepiapterin manufacturer, verifying that the manufacturing facility of a Sepiapterin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Sepiapterin APIs or Sepiapterin finished pharmaceutical products to another nation, regulatory agencies frequently require a Sepiapterin WC (written confirmation) as part of the regulatory process.
click here to find a list of Sepiapterin suppliers with Written Confirmation (WC) on PharmaCompass.
Sepiapterin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Sepiapterin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Sepiapterin GMP manufacturer or Sepiapterin GMP API supplier for your needs.
A Sepiapterin CoA (Certificate of Analysis) is a formal document that attests to Sepiapterin's compliance with Sepiapterin specifications and serves as a tool for batch-level quality control.
Sepiapterin CoA mostly includes findings from lab analyses of a specific batch. For each Sepiapterin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Sepiapterin may be tested according to a variety of international standards, such as European Pharmacopoeia (Sepiapterin EP), Sepiapterin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Sepiapterin USP).

