By PharmaCompass
2025-12-04
Impressions: 40
November saw several big ticket acquisitions across the consumer health and biopharma space, including the US$ 48.7 billion acquisition of Johnson & Johnson’s consumer health unit Kenvue by Kimberly-Clark and Pfizer’s up to US$ 10 billion acquisition of obesity-focused biotech Metsera. Additionally, the trend of companies announcing capacity expansions continued unabated.
The month saw several key drug approvals by the US Food and Drug Administration (FDA), including Kura Oncology-Kyowa Kirin’s Komzifti (ziftomenib) for treating adults with relapsed or refractory acute myeloid leukemia (AML) with an NPM1 mutation.
The indices rose substantially during the month. The Nasdaq Biotechnology Index (NBI) rose 8.85 percent, from 5,344.91 at the end of October to 5,818.03 by November-end. The SPDR S&P Biotech ETF (XBI) gained 10.97 percent from 110.98 to 123.16. The S&P Biotechnology Select Industry Index (SPSIBI) also advanced 9.08 percent — from 8,789.93 to 9,588.09.
Access the Pipeline Prospector Dashboard for November 2025 Newsmakers (Free Excel)
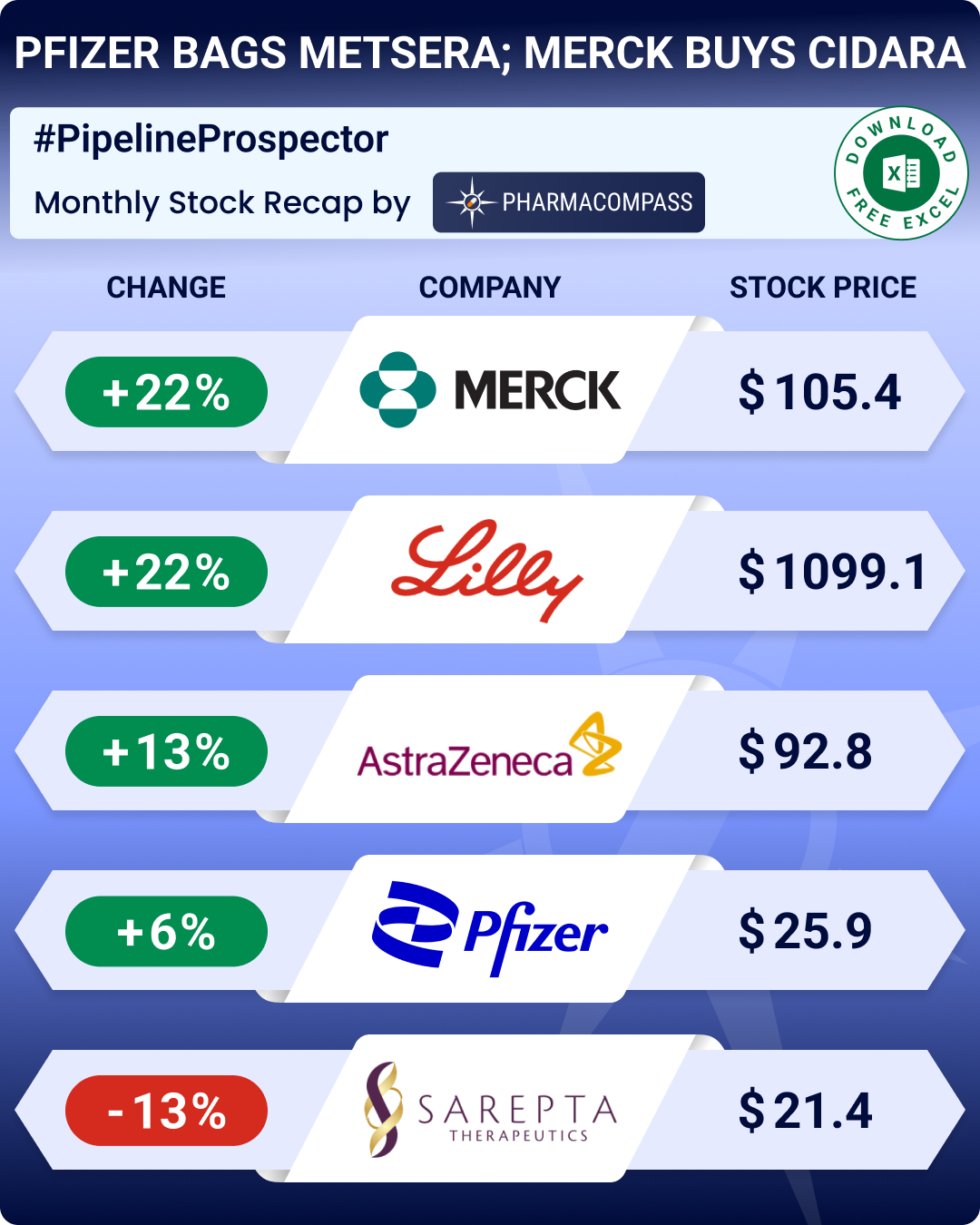
Pfizer clinches up to US$ 10 bn deal for Metsera; Merck to buy Cidara for US$ 9.2 bn
Pfizer inked an agreement worth up to US$ 10 billion to acquire obesity-focused biotech Metsera. The deal ended a competitive bidding process between Pfizer and Novo Nordisk. Pfizer had filed lawsuits, alleging that Metsera breached an earlier merger agreement. Metsera accepted Pfizer’s revised offer after raising concerns about potential antitrust risks associated with Novo’s competing bid.
In early November, Kimberly-Clark announced the acquisition of Johnson & Johnson’s consumer health unit — Kenvue — in a cash-and-stock deal valued at US$ 48.7 billion. The merger will create a consumer health company with expected annual revenue of about US$ 32 billion and a portfolio of highly-valued brands, including Tylenol (paracetamol), Neutrogena, Aveeno, and Listerine.
Merck announced the acquisition of Cidara Therapeutics in a deal valued at US$ 9.2 billion. The acquisition gives Merck access to an experimental influenza antiviral designed to provide season-long protection with a single dose. Its stock rose 22 percent in November.
Johnson & Johnson is buying Halda Therapeutics for US$ 3.05 billion in cash. Halda, a Connecticut-based biotech, adds early-stage cancer programs to J&J’s pipeline, including a lead candidate in prostate cancer.
Like Pfizer and Novo Nordisk, Lundbeck and Alkermes were also embroiled in a bidding war for Avadel Pharmaceuticals. Lundbeck later withdrew from the race, clearing the way for Alkermes, which eventually agreed to acquire Avadel for US$ 2.37 billion.
Drugmakers continued to announce plans to expand capacities. Eli Lilly (whose stock rose 22 percent in November) announced plans to invest US$ 3 billion to build an oral medicines manufacturing site in the Netherlands to support its experimental GLP-1 pill, orforglipron. It will also invest more than US$ 1.2 billion to expand its facility in Puerto Rico.
AstraZeneca said it will invest US$ 2 billion to expand its manufacturing capacity in Maryland (US). And Novartis said it will set up a flagship manufacturing hub in North Carolina, post a trade agreement that lowers US tariff rates on Swiss imports.
Access the Pipeline Prospector Dashboard for November 2025 Newsmakers (Free Excel)
FDA approves Komzifti for leukemia; Bayer’s Hyrnuo wins accelerated approval for lung cancer
FDA approved Komzifti (ziftomenib), a once-daily pill from Kura Oncology and Japan’s Kyowa Kirin, for adults with relapsed or refractory acute myeloid leukemia (AML) with an NPM1 mutation.
The agency also expanded the approval of Epkinly (epcoritamab), developed by AbbVie and Genmab, for use as a second-line treatment of relapsed or refractory follicular lymphoma.
The agency also granted accelerated approval to Bayer’s Hyrnuo (sevabertinib) for people with non-squamous non-small cell lung cancer (NSCLC) that has recurred or spread and carries a specific HER2 mutation.
Moreover, FDA approved UCB’s Kygevvi (doxecitine and doxribtimine), the first treatment for thymidine kinase 2 deficiency (TK2d), a rare inherited mitochondrial disorder that leads to progressive muscle weakness and respiratory complications.
Further, FDA approved Redemplo (plozasiran sodium), Arrowhead Pharmaceuticals’ first marketed drug for adults with familial chylomicronemia syndrome (FCS), a rare genetic disorder that causes extremely high levels of triglycerides in the blood that can cause acute pancreatitis. During the month, Arrowhead’s stock price went up by 31 percent.
FDA also granted accelerated approval to Otsuka’s Voyxact (sibeprenlimab-szsi) to help reduce excess urinary protein in adults with IgA nephropathy, a chronic kidney disease.
Separately, FDA approved Novartis’ gene-replacement therapy Itvisma (onasemnogene abeparvovec-brve) for patients aged two years and older with spinal muscular atrophy (SMA) caused by an SMN1 mutation. Itvisma uses the same active ingredient as Zolgensma and is the first approved gene therapy for a broader SMA population.
Other than these, the regulator approved the Padcev (enfortumab vedotin) and Keytruda (pembrolizumab) regimen for people with muscle-invasive bladder cancer who cannot receive cisplatin-based chemotherapy. The combination is the first and only approved regimen that can be used before and after surgery.
Access the Pipeline Prospector Dashboard for November 2025 Newsmakers (Free Excel)
Bayer’s asundexian shows benefit in strokes; Novo’s Rybelsus fails Alzheimer’s trials
The month saw several trial wins. For instance, Bayer reported positive phase 3 results for asundexian, an experimental blood thinner that lowered the risk of repeated strokes without increasing major bleeding.
In another phase 3 trial, Amgen’s Repatha (evolocumab) reduced major cardiovascular events in adults without prior heart attack or stroke when added to statins or other therapies.
Cogent Biosciences (stock up 146 percent in November) reported strong phase 3 results for bezuclastinib in combination with sunitinib in patients with gastrointestinal stromal tumor (GIST) whose disease had progressed after earlier treatment.
In trial failures, Novo Nordisk’s oral semaglutide pill, Rybelsus, failed to slow Alzheimer’s disease progression in two large phase 3 trials. The studies showed no benefit over placebo in more than 3,800 people with early-stage disease.
The agency also paused two phase 3 studies of Intellia’s gene-editing therapy nexiguran ziclumeran after a patient developed severe liver problems. The share price of the company fell by 33 percent over the month.
November wasn’t a good month for Sarepta (its stock was down 13 percent). The company said its late-stage trial of casimersen and golodirsen for Duchenne muscular dystrophy did not meet its main goal. Moreover, FDA added a boxed warning to Sarepta’s gene therapy Elevidys (delandistrogene moxeparvovec-rokl) following the deaths of two non-ambulatory children from acute liver failure.
Access the Pipeline Prospector Dashboard for November 2025 Newsmakers (Free Excel)
Our view
After a few difficult years marked by stock-market volatility and disruptions in global supply chains, the pharmaceutical sector is finally showing signs of sustained growth and stability. The uptick in big-ticket M&A activity is a clear indicator of the industry’s confidence. These are all huge positives as we move closer to 2026.
Access the Pipeline Prospector Dashboard for November 2025 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in November 2025
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : LILLY HITS US$ 1 TN; PFIZER BAGS METSERA by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”