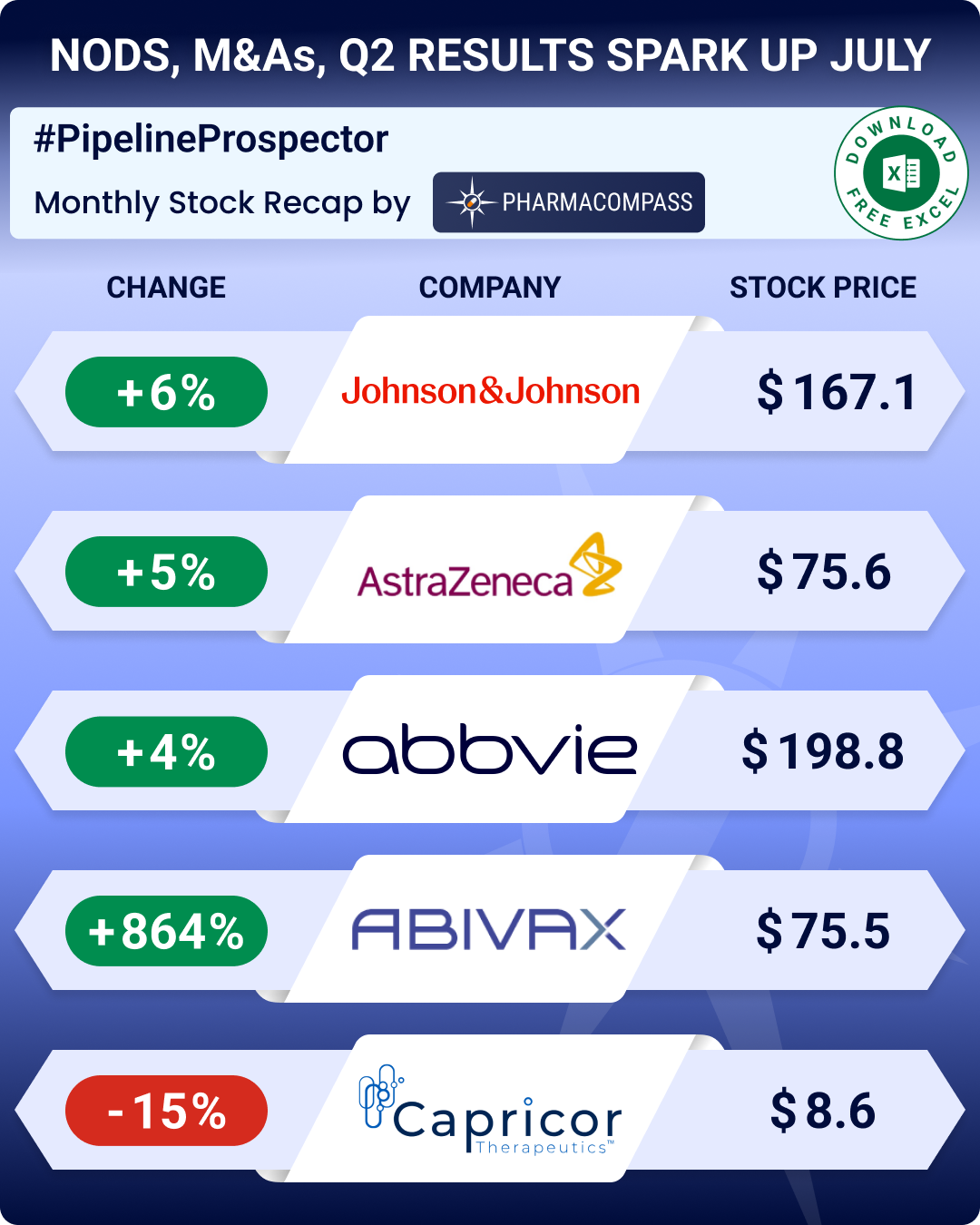
By PharmaCompass
2022-06-23
Impressions: 2783
Skin is the largest organ in our body that protects our internal organs from bacteria and injury. Most of us have experienced a dermatological condition at some point in life. However, of late, there has been an alarming rise in the prevalence of skin diseases such as acne, atopic dermatitis (AD), psoriasis and rosacea due to factors like environmental pollution, stress, lifestyle-related factors, etc. As a result, dermatology, or the branch of medicine that deals with skin and various skin diseases, has seen impressive growth.
According to estimates, the global dermatological market is poised to expand at a compounded annual growth rate (CAGR) of 11.5 percent, increasing it from US$ 19.9 billion in 2020 to US$ 59.3 billion by 2030.
Over the last few years, dermatology has witnessed technological advancements, new drug approvals and several deals. The big players in the field include Galderma, Almirall, Amgen, Sanofi, AbbVie, Allergan, Eli Lilly, GSK, Janssen and Novartis.
Access the Dermatology Newsmakers Dashboard
Vaccines for acne, personalized meds, CRISPR to revolutionize dermatology
Several technological breakthroughs have enabled early detection of skin diseases. Last month, Virginia-based AMPEL BioSolutions announced a breakthrough in precision and personalized medicine that can revolutionize the way doctors treat lupus, psoriasis, atopic dermatitis and scleroderma. This machine-learning approach characterizes disease activity from gene expression data obtained from the patient’s skin biopsies. It holds the potential to transform the way doctors treat chronic skin diseases by diagnosing and characterizing the precise molecular abnormalities and treating skin diseases before the damage begins.
Work is also being undertaken to develop a vaccine against acne, a common bacterial disease affecting adolescents and young adults. In December last year, Sanofi had acquired Origimm Biotechnology and gained access to ORI-001, the first-ever vaccine candidate against acne. The vaccine targets a type of bacteria living in our skin and stops it from producing toxins that cause inflammation. The phase 1/2 trial for the vaccine is slated to begin in 2023.
Similarly, GSK signed a potential US$ 224 million deal with Eligo Bioscience last year for the latter’s Eligobiotics technology that uses the CRISPR system (a technology that can edit genes) to disable inflammation inducing genes. Players like Abeona Therapeutics, Aegle Therapeutics and Castle Creek Biosciences are also leveraging cell and gene therapy to treat epidermolysis bullosa, a rare inherited skin disorder that causes the skin to become very fragile.
There has been a rise in approvals of dermatological products in recent years, driving market growth. Only this month, Eli Lilly and Incyte announced FDA’s approval of Olumiant (baricitinib) as the first-ever systemic treatment for alopecia areata, an autoimmune disorder that triggers sudden hair loss. Olumiant was first approved in 2018 for rheumatoid arthritis.
In April 2022, FDA approved Sol-Gel Technologies and Galderma’s Epsolay topical cream for the treatment of inflammatory lesions of rosacea in adults. Rosacea is a condition that causes redness and often small, red, pus-filled bumps on the face. In July 2021, FDA had approved Twyneo cream, the first fixed-dose combination of tretinoin and benzoyl peroxide to treat acne or inflammatory lesions of rosacea.
Access the Dermatology Newsmakers Dashboard
Pfizer’s Cibinqo, AbbVie’s Rinvoq, Sanofi’s Dupixent bag FDA approvals for eczema
Atopic dermatitis (AD), also known as eczema, is characterized by patches of dry, inflamed and itchy skin. It can appear at any age, but is particularly common in childhood.
Janus kinases (JAKs) are intracellular enzymes that often play a role in pro-inflammatory cellular responses. JAK inhibitors are a new class of drugs that treat atopic dermatitis.
On January 14, FDA approved Pfizer’s Cibinqo (abrocitinib), a JAK inhibitor for adults with moderate-to-severe AD. Pfizer has pegged Cibinqo’s peak sales at US$ 3 billion. The same day, FDA also approved AbbVie’s Rinvoq (upadacitinib) tablet, another JAK inhibitor, for the treatment of moderate-to-severe AD in adults and children 12 years and older. AbbVie is expecting 2025 risk-adjusted sales of over US$ 7.5 billion for Rinvoq. Both these drugs were authorized in Europe last year.
In January 2021, Sanofi acquired Kymab for up to US$ 1.5 billion. Through this deal, Sanofi will gain access to Kymab’s candidate in phase 2 trial — KY1005 (amlitelimab) — a monoclonal antibody meant to treat a wide range of inflammatory disorders, including AD and cancer. This month, Sanofi and Regeneron’s Dupixent (dupilumab) became the first and only biologic medicine in the US to treat moderate-to-severe eczema in young children.
LEO Pharma received US approval for the injectable drug Adbry (tralokinumab-ldrm) as the first and only treatment targeting IL-13 for adults with moderate-to-severe AD in December 2021. IL-13 is a cytokine used in allergic and other inflammatory conditions. The drug — Adtralza (tralokinumab) — was authorized by the European Commission in June last year. Back in 2016, LEO Pharma had signed an exclusive global licensing agreement with AstraZeneca for tralokinumab.
Eli Lilly also announced positive top-line results from a phase 3 trial for lebrikizumab, an IL-13 monoclonal antibody. Lilly plans to submit a biologics license application (BLA) to the FDA for lebrikizumab in AD in the second half of 2022.
Amgen and Kyowa Kirin have announced a potential US$ 1.2 billion deal to jointly develop and commercialize KHK4083 (rocatinlimab), which is currently in a phase 3 trial. KHK4083 is a human monoclonal antibody based on Kyowa Kirin’s patented technology for the treatment of moderate-to-severe AD. AstraZeneca is also using the same Kyowa Kirin technology in Fasenra (benralizumab), which is already approved in asthma, and is currently in phase 2 trials for AD.
Access the Dermatology Newsmakers Dashboard
FDA approves Amgen’s Otezla, Dermavant’s Vtama for plaque psoriasis
Psoriasis, an immune-mediated inflammatory disease, also saw several developments. The most common form of psoriasis is plaque psoriasis, characterized by dry, itchy and raised skin patches covered with scales. In December 2021, FDA approved Amgen’s Otezla (apremilast) for the treatment of plaque psoriasis. The drug brought in US$ 451 million in the first quarter of 2022.
Amgen’s second drug to treat the same condition – Enbrel – made nearly twice the amount, or US$ 862 million, during the last quarter. Samsung Bioepis has a biosimilar referencing Enbrel, known as Benepali (etanercept), which is a pre-filled syringe injection. In January this year, Samsung Biologics agreed to shell out US$ 2.3 billion to take over Biogen’s stake in Samsung Bioepis, a joint venture set up in 2021 between Biogen and Samsung to develop and manufacture biosimilars.
In May, Dermavant Sciences received an FDA approval for Vtama (tapinarof), a topical cream to treat plaque psoriasis in adults. Vtama is the first steroid-free topical therapy in its class.
In negative news, FDA issued a complete response letter (CRL) last month to UCB’s Bimzelx (bimekizumab), meant for the treatment of adults with moderate-to-severe plaque psoriasis, over facility inspection issues. Bimekizumab is already approved in Europe and Japan.
People with psoriasis often experience joint inflammation that produces symptoms of arthritis, a condition known as psoriatic arthritis. BMS’ deucravacitinib is currently under regulatory review in multiple regions, including the US, Europe and Japan. If approved, it will become the first selective allosteric TYK2 inhibitor to treat moderate-to-severe plaque psoriasis and will compete against Amgen’s Otezla. TYK2, short for tyrosine kinase 2, is a member of the JAK family. The therapeutic value of targeting this pathway in autoimmune diseases is supported by human genetics. FDA will take a decision on deucravacitinib in September. BMS estimates deucravacitinib to reach US$ 4 billion sales in peak sales.
In October 2021, MoonLake Immunotherapeutics and Helix Acquisition had entered into a business combination agreement focused on advancing tri-specific nanobody sonelokimab, which is in phase 2 trials, for the treatment of psoriasis, along with other skin and joint diseases.
In 2021, Protagonist Therapeutics revised the agreement with Janssen Pharmaceutical to increase the deal amount to US$ 987.5 million. Back in 2017, the two companies had announced the deal to develop PN-235, the first oral IL-23 receptor antagonist currently in phase 2 for plaque psoriasis. In March this year, Protagonist received US$ 25 million in milestone payment. If approved, the drug will become the first targeted oral therapy to treat plaque psoriasis.
Access the Dermatology Newsmakers Dashboard
Our view
Growth in skin diseases, technological advancements and awareness campaigns are spurring growth in the market for dermatological products. The pandemic saw an increase in the use of teledermatology. Mobile apps, telemedicine, machine learning algorithms, nanomedicines and artificial intelligence are increasingly being used by doctors to treat skin diseases.
JAK inhibitors, such as Pfizer’s Cibinqo, Abbie’s Rinvoq and Lilly’s Olumiant, are emerging as a new class of drugs. However, data has linked these therapies to an increased risk for cardiovascular events and cancer. As a result, interest has shifted to the more selective inhibition of TYK2, such as BMS’ deucravacitinib.
In terms of revenue, North America brought in the largest revenue share for dermatological drugs in 2021, cornering more than 35 percent of the market. Europe is the second-largest market, with more than 30 percent share in revenue in 2021. However, the Asia Pacific market is likely to expand at the highest CAGR of 12.2 percent until 2030. Given these projections, we are likely to see a lot more deals, drug approvals and technological advancements in the years to come.
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Dermatology Newsmakers by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”