06 Sep 2025
// PRESS RELEASE
14 Aug 2025
// PRESS RELEASE
13 Aug 2025
// PRESS RELEASE
 KEY PRODUCTS
KEY PRODUCTS
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
About
Products listed herein may not be available for commercial use in countries where any relevant third-party intellectual property is in force.
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth #H9.1A48
28-30 October, 2025
Industry Trade Show
Attending
25-27 November, 2025
CPhI Middle EastCPhI Middle East
Industry Trade Show
Attending
08-10 December, 2025
CONTACT DETAILS





Events
Webinars & Exhibitions
CPhI WW FrankfurtCPhI WW Frankfurt
Industry Trade Show
Booth #H9.1A48
28-30 October, 2025
Industry Trade Show
Attending
25-27 November, 2025
CPhI Middle EastCPhI Middle East
Industry Trade Show
Attending
08-10 December, 2025
VLOG #PharmaReel
CORPORATE CONTENT #SupplierSpotlight
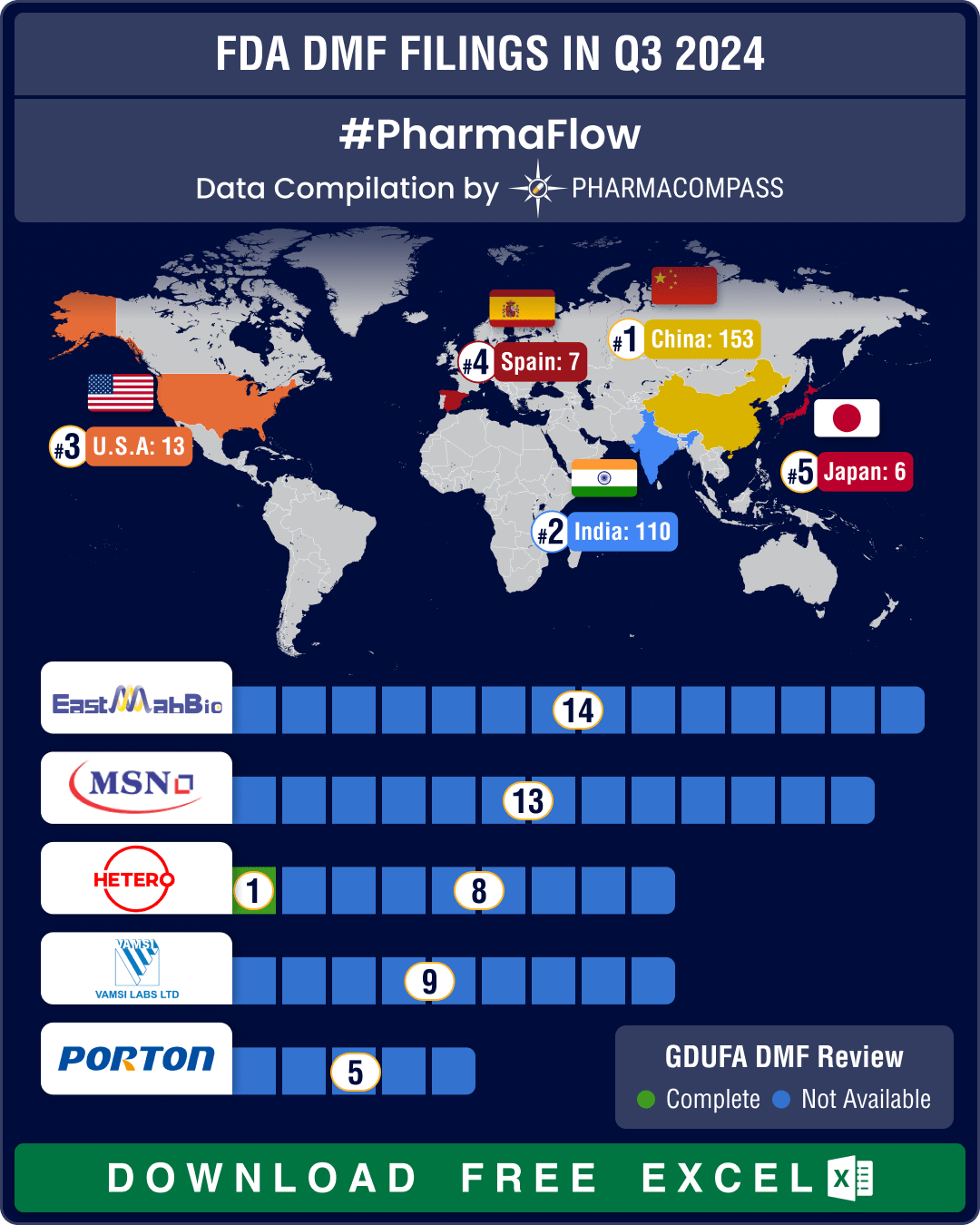
https://www.pharmacompass.com/radio-compass-blog/dmf-filings-hit-all-time-high-in-q3-2024-china-tops-list-with-58-increase-in-type-ii-submissions

06 Sep 2025
// PRESS RELEASE
https://granulesindia.com//wp-content/uploads/2025/09/Granules-India-and-NIPER-S.A.S-Nagar-Launch-Centre-of-Excellence-for-Sustainable-Pharma-Innovation.pdf

14 Aug 2025
// PRESS RELEASE
https://granulesindia.com//wp-content/uploads/2025/08/Press-Release-Q1-FY26.pdf

13 Aug 2025
// PRESS RELEASE
https://granulesindia.com//wp-content/uploads/2025/08/Press-Release-Q1-FY26.pdf

27 Jul 2025
// INDPHARMAPOST
https://www.indianpharmapost.com/people/granules-india-ceo-k-v-sitaram-rao-resigns-17476

29 May 2025
// PRESS RELEASE
https://granulesindia.com/wp-content/uploads/2025/05/Press-Release-Q4-FY25.pdf

14 Apr 2025
// PRESS RELEASE
https://granulesindia.com/wp-content/uploads/2025/04/Granules-India-Announces-Closing-of-Acquisition-of-Senn-Chemicals-Strengthening-Capabilities-in-Peptide-Therapeutics-and-CDMO-Services.pdf
Details:
FDA approved Lisdexamfetamine Dimesylate Capsules, a bioequivalent and therapeutically equivalent to the reference listed drug, Vyvanse for the treatment of Attention Deficit Hyperactivity Disorder.
Lead Product(s): Lisdexamfetamine Dimesylate,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Vyvanse-Generic
Study Phase: Approved FDFProduct Type: Controlled Substance
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 30, 2025
Lead Product(s) : Lisdexamfetamine Dimesylate,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules Strengthens ADHD Portfolio with FDA Approval for Lisdexamfetamine Dimesylate
Details : FDA approved Lisdexamfetamine Dimesylate Capsules, a bioequivalent and therapeutically equivalent to the reference listed drug, Vyvanse for the treatment of Attention Deficit Hyperactivity Disorder.
Product Name : Vyvanse-Generic
Product Type : Controlled Substance
Upfront Cash : Inapplicable
January 30, 2025
Details:
FDA approved Lisdexamfetamine Dimesylate Tablets, a bioequivalent and therapeutically equivalent to the reference listed drug, Vyvanse for the treatment of Attention Deficit Hyperactivity Disorder.
Lead Product(s): Lisdexamfetamine Dimesylate,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Vyvanse-Generic
Study Phase: Approved FDFProduct Type: Controlled Substance
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 17, 2024
Lead Product(s) : Lisdexamfetamine Dimesylate,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules India Limited Announces FDA Approval for ADHD Treatment
Details : FDA approved Lisdexamfetamine Dimesylate Tablets, a bioequivalent and therapeutically equivalent to the reference listed drug, Vyvanse for the treatment of Attention Deficit Hyperactivity Disorder.
Product Name : Vyvanse-Generic
Product Type : Controlled Substance
Upfront Cash : Inapplicable
December 17, 2024
Details:
FDA approved bupropion hydrochloride extended-release tablets USP (SR) are bioequivalent and therapeutically equivalent to Wellbutrin SR sustained-release tablets.
Lead Product(s): Bupropion Hydrochloride,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Wellbutrin SR-Generic
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 18, 2024
Lead Product(s) : Bupropion Hydrochloride,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules Announces ANDA Approval for Bupropion Hydrochloride Extended-Release Tablets
Details : FDA approved bupropion hydrochloride extended-release tablets USP (SR) are bioequivalent and therapeutically equivalent to Wellbutrin SR sustained-release tablets.
Product Name : Wellbutrin SR-Generic
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
October 18, 2024
Details:
Desyrel-Generic (trazodone hydrochloride) is a selective serotonin reuptake inhibitor indicated for the treatment of patients with major depressive disorder.
Lead Product(s): Trazodone Hydrochloride,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Desyrel-Generic
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 08, 2024
Lead Product(s) : Trazodone Hydrochloride,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules India Gains ANDA approval for Trazodone Tablets
Details : Desyrel-Generic (trazodone hydrochloride) is a selective serotonin reuptake inhibitor indicated for the treatment of patients with major depressive disorder.
Product Name : Desyrel-Generic
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 08, 2024
Details:
Cuvposa is an anticholinergic indicated to reduce chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling.
Lead Product(s): Glycopyrronium Bromide,Inapplicable
Therapeutic Area: Neurology Brand Name: Cuvposa-Generic
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 20, 2024
Lead Product(s) : Glycopyrronium Bromide,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules India Gets USFDA Nod for Generic Glycopyrrolate Oral Solution
Details : Cuvposa is an anticholinergic indicated to reduce chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling.
Product Name : Cuvposa-Generic
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
August 20, 2024
Details:
Pantoprazole Sodium Delayed-Release tablets are indicated for short-term treatment of Erosive Esophagitis Associated with GERD and Pathological Hypersecretory Conditions.
Lead Product(s): Pantoprazole Sodium,Inapplicable
Therapeutic Area: Gastroenterology Brand Name: Protonix-Generic
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 13, 2023
Lead Product(s) : Pantoprazole Sodium,Inapplicable
Therapeutic Area : Gastroenterology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules India Limited Received ANDA Approval for Pantoprazole Sod Delayed-Release Tablets
Details : Pantoprazole Sodium Delayed-Release tablets are indicated for short-term treatment of Erosive Esophagitis Associated with GERD and Pathological Hypersecretory Conditions.
Product Name : Protonix-Generic
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
December 13, 2023
Details:
Esomeprazole magnesium delayed-release capsules is a proton pump inhibitor indicated for the treatment of gastroesophageal reflux disease and NSAID-associated gastric ulcer.
Lead Product(s): Esomeprazole Magnesium,Inapplicable
Therapeutic Area: Gastroenterology Brand Name: Nexium-Generic
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 19, 2023
Lead Product(s) : Esomeprazole Magnesium,Inapplicable
Therapeutic Area : Gastroenterology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules India Gets USFDA Nod for Generic Drug Used for Short-term Treatment of Heartburn
Details : Esomeprazole magnesium delayed-release capsules is a proton pump inhibitor indicated for the treatment of gastroesophageal reflux disease and NSAID-associated gastric ulcer.
Product Name : Nexium-Generic
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
October 19, 2023
Details:
Generic of Losartan potassium and hydrochlorothiazide tablets are been approved by FDA, indicated for hypertension to lower blood pressure and to reduce the risk of stroke.
Lead Product(s): Losartan Potassium,Hydrochlorothiazide
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Cozaar-Generic
Study Phase: Approved FDFProduct Type: Hormone
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 30, 2023
Lead Product(s) : Losartan Potassium,Hydrochlorothiazide
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules India Limited Received ANDA Approval for Losartan and Hydrochlorothiazide Tablets
Details : Generic of Losartan potassium and hydrochlorothiazide tablets are been approved by FDA, indicated for hypertension to lower blood pressure and to reduce the risk of stroke.
Product Name : Cozaar-Generic
Product Type : Hormone
Upfront Cash : Inapplicable
September 30, 2023
Details:
The company has received approval from the US Food and Drug Administration (USFDA) for generic version for Acetaminophen and Ibuprofen tablets (250 mg/125 mg) indicated for pain and inflammation.
Lead Product(s): Ibuprofen,Inapplicable
Therapeutic Area: Neurology Brand Name: Advil-Generic
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 14, 2023
Lead Product(s) : Ibuprofen,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Approves Granules' OTC Equivalent Of Advil Dual Action Tablets
Details : The company has received approval from the US Food and Drug Administration (USFDA) for generic version for Acetaminophen and Ibuprofen tablets (250 mg/125 mg) indicated for pain and inflammation.
Product Name : Advil-Generic
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 14, 2023
Details:
FDA approved generic of Levetiracetam for seizures. It prevents seizure activity via selective inhibition of hypersynchronized epileptiform burst firing without affecting normal neuronal transmission.
Lead Product(s): Levetiracetam,Inapplicable
Therapeutic Area: Neurology Brand Name: Keppra-Generic
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 14, 2023
Lead Product(s) : Levetiracetam,Inapplicable
Therapeutic Area : Neurology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Granules India Limited Receives ANDA Approval for Levetiracetam Tablets in record 9 months
Details : FDA approved generic of Levetiracetam for seizures. It prevents seizure activity via selective inhibition of hypersynchronized epileptiform burst firing without affecting normal neuronal transmission.
Product Name : Keppra-Generic
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
June 14, 2023
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Inspections and registrations
ABOUT THIS PAGE
Granules India Limited is a supplier offers 53 products (APIs, Excipients or Intermediates).
Find a price of Metformin bulk with DMF, CEP, JDMF, WC offered by Granules India Limited
Find a price of Paracetamol bulk with DMF, CEP, JDMF, WC offered by Granules India Limited
Find a price of Guaifenesin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Metformin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Rifaximin bulk with DMF, CEP, WC offered by Granules India Limited
Find a price of Atazanavir Sulfate bulk with DMF, CEP offered by Granules India Limited
Find a price of Cetirizine Dihydrochloride bulk with DMF, WC offered by Granules India Limited
Find a price of Dapagliflozin bulk with DMF, WC offered by Granules India Limited
Find a price of Dimethyl Fumarate bulk with DMF, WC offered by Granules India Limited
Find a price of Empagliflozin bulk with DMF, WC offered by Granules India Limited
Find a price of Esomeprazole Magnesium bulk with DMF, CEP offered by Granules India Limited
Find a price of Fexofenadine Hydrochloride bulk with DMF, WC offered by Granules India Limited
Find a price of Levetiracetam bulk with DMF, CEP offered by Granules India Limited
Find a price of Levocetirizine Dihydrochloride bulk with DMF, WC offered by Granules India Limited
Find a price of Losartan Potassium bulk with DMF, WC offered by Granules India Limited
Find a price of Methocarbamol bulk with DMF, WC offered by Granules India Limited
Find a price of Omeprazole bulk with DMF, CEP offered by Granules India Limited
Find a price of Omeprazole Magnesium bulk with DMF, WC offered by Granules India Limited
Find a price of Pantoprazole Sodium bulk with DMF, CEP offered by Granules India Limited
Find a price of Paracetamol bulk with DMF, WC offered by Granules India Limited
Find a price of Pirfenidone bulk with DMF, WC offered by Granules India Limited
Find a price of Rifaximin bulk with DMF, CEP offered by Granules India Limited
Find a price of Sorafenib bulk with DMF, CEP offered by Granules India Limited
Find a price of Trazodone Hydrochloride bulk with DMF, CEP offered by Granules India Limited
Find a price of Eltrombopag bulk with DMF offered by Granules India Limited
Find a price of Guaifenesin bulk with DMF offered by Granules India Limited
Find a price of Metoprolol Succinate bulk with DMF offered by Granules India Limited
Find a price of Bupropion Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Dasatinib bulk with DMF offered by Granules India Limited
Find a price of Esomeprazole Magnesium bulk with DMF offered by Granules India Limited
Find a price of Ibuprofen bulk with DMF offered by Granules India Limited
Find a price of Linagliptin bulk with DMF offered by Granules India Limited
Find a price of Lisdexamfetamine Dimesylate bulk with DMF offered by Granules India Limited
Find a price of Losartan Potassium bulk with DMF offered by Granules India Limited
Find a price of Nilotinib bulk with DMF offered by Granules India Limited
Find a price of Paracetamol bulk with DMF offered by Granules India Limited
Find a price of Pazopanib Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Pirfenidone bulk with WC offered by Granules India Limited
Find a price of Prazosin Hydrochloride bulk with DMF offered by Granules India Limited
Find a price of Tenofovir Disoproxil Succinate bulk with WC offered by Granules India Limited
Find a price of Vigabatrin bulk with DMF offered by Granules India Limited
Find a price of Metformin bulk offered by Granules India Limited
Find a price of Methocarbamol bulk offered by Granules India Limited
Find a price of Avatrombopag Maleate bulk offered by Granules India Limited
Find a price of Cabozantinib bulk offered by Granules India Limited
Find a price of Elacestrant bulk offered by Granules India Limited
Find a price of Eltrombopag bulk offered by Granules India Limited
Find a price of Gabapentin bulk offered by Granules India Limited
Find a price of Guaifenesin bulk offered by Granules India Limited
Find a price of Ibuprofen bulk offered by Granules India Limited
Find a price of Lapatinib Ditosylate bulk offered by Granules India Limited
Find a price of Levocetirizine Dihydrochloride bulk offered by Granules India Limited
Find a price of Paracetamol bulk offered by Granules India Limited