
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
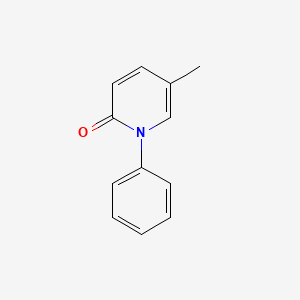

1. 1-phenyl-5-(trideuteriomethyl)-1,2-dihydropyridin-2-one
2. 5-methyl-1-phenyl-2-(1h)-pyridone
3. Deskar
4. Deupirfenidone
5. Esbriet
1. 53179-13-8
2. 5-methyl-1-phenylpyridin-2(1h)-one
3. Esbriet
4. Amr-69
5. Deskar
6. Pirespa
7. 5-methyl-1-phenyl-2-(1h)-pyridone
8. Pirfenidonum
9. Pirfenidona
10. 2(1h)-pyridinone, 5-methyl-1-phenyl-
11. 5-methyl-1-phenylpyridin-2-one
12. 5-methyl-1-phenyl-2(1h)-pyridinone
13. 5-methyl-1-phenyl-2(1h)-pyridone
14. Amr 69
15. 5-methyl-1-phenyl-1h-pyridin-2-one
16. 5-methyl-1-phenyl-2-pyridinone
17. Mfcd00866047
18. D7nld2jx7u
19. Nsc-748456
20. Amr69
21. Chebi:32016
22. S-7701
23. Ncgc00015806-03
24. Dsstox_cid_25183
25. Dsstox_rid_80731
26. Dsstox_gsid_45183
27. Pirfenidone [usan:inn]
28. Pirfenidonum [inn-latin]
29. Pirfenidona [inn-spanish]
30. Cas-53179-13-8
31. Sr-01000076061
32. Unii-d7nld2jx7u
33. Brn 1526549
34. Prfendone
35. Pirfenidone (jan/usan/inn)
36. 5-methyl-1-phenyl-pyridin-2-one
37. Pirfenidone-[d3]
38. Esbriet (tn)
39. Pirfenidone- Bio-x
40. Pirfenidone(amr69)
41. Ks-5041
42. 2(1h)-pyridone, 5-methyl-1-phenyl-
43. Tocris-1093
44. Pirfenidone [mi]
45. Lopac-p-2116
46. Pirfenidone [inn]
47. Pirfenidone [jan]
48. F-647
49. Pirfenidone [usan]
50. P 2116
51. Pirfenidone [vandf]
52. Schembl4708
53. Pirfenidone [mart.]
54. Lopac0_000907
55. Pirfenidone [who-dd]
56. 5-21-07-00197 (beilstein Handbook Reference)
57. Mls000860042
58. Pirfenidone [ema Epar]
59. 5-methyl-1-phenyl-2-pyridone
60. Gtpl7532
61. Zinc1958
62. Chembl1256391
63. Dtxsid4045183
64. 1-phenyl-5-methyl-2-pyridinone
65. Hsdb 8340
66. Pirfenidone, >=97% (hplc)
67. Pirfenidone [orange Book]
68. Bio1_000397
69. Bio1_000886
70. Bio1_001375
71. Hms2234g24
72. Hms3262f16
73. Hms3267i06
74. Hms3372a08
75. Hms3412g13
76. Hms3651p08
77. Hms3676g13
78. Pirfenidone [ep Monograph]
79. Bcp04473
80. Esbriet Component Pirfenidone
81. Hy-b0673
82. Tox21 110225
83. Tox21_110225
84. Tox21_500907
85. Bdbm50005201
86. Nsc748456
87. S2907
88. Akos006273697
89. S-7701,amr-69
90. Tox21_110225_1
91. Ab07515
92. Ac-6797
93. Am84939
94. Ccg-204989
95. Db04951
96. Lp00907
97. Nsc 748456
98. Pirfenidone Component Of Esbriet
99. Sdccgsbi-0050882.p002
100. Ncgc00015806-01
101. Ncgc00015806-02
102. Ncgc00015806-04
103. Ncgc00015806-05
104. Ncgc00015806-06
105. Ncgc00015806-17
106. Ncgc00024992-01
107. Ncgc00024992-02
108. Ncgc00024992-03
109. Ncgc00261592-01
110. Bm164275
111. Smr000326900
112. Sy034783
113. B2288
114. Eu-0100907
115. Ft-0602686
116. Ft-0672092
117. P1871
118. Sw220156-1
119. D01583
120. 5-methyl-n-phenyl-2-1h-pyridone [pirfenidone]
121. 179p138
122. A829431
123. J-523979
124. Q2060696
125. Sr-01000076061-1
126. Sr-01000076061-3
127. 5-methyl-n-phenyl-2-1h-pyridone-d5(pirfenidone-d5)
128. Brd-k96862998-001-03-1
129. Brd-k96862998-001-09-8
130. Pirfenidone, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 185.22 g/mol |
|---|---|
| Molecular Formula | C12H11NO |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 185.084063974 g/mol |
| Monoisotopic Mass | 185.084063974 g/mol |
| Topological Polar Surface Area | 20.3 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 285 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Pirfenidone is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 6, 2016: https://clinicaltrials.gov/search/intervention=pirfenidone
Esbriet is indicated for the treatment of idiopathic pulmonary fibrosis (IPF). /Included in US product label/
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
/EXPL THER/ Left ventricular remodeling is a frequent complication of hypertension with no therapeutic treatment available for the subsequent onset of myocardial fibrosis. Pirfenidone is an antifibrotic small-molecular-size drug with anti-inflammatory properties that is used as a treatment for fibrotic diseases, but its effects on hypertension-induced myocardial fibrosis are unknown. Therefore, we tested whether pirfenidone could ameliorate hypertension-induced left ventricular remodeling and whether hypertension-induced NLRP3 (Nod-like receptor pyrin domain containing 3), a critical protein in NLRP3 inflammasome formation, is involved in the therapeutic mechanism. A TAC-induced mouse model of hypertension and left ventricular hypertrophy was treated with pirfenidone, and survival, collagen deposition by histopathologic examination, heart function by echocardiography, concentrations of fibrosis-related inflammatory cytokines TGF-beta1, IL-1beta in heart homogenate and in vitro cell cultures by ELISA, levels of ROS and inflammatory cells by flow cytometry, and levels of NLRP3 by Western blotting and immunohistochemistry were measured. Pirfenidone increased the survival rate and attenuated myocardial fibrosis and inflammatory mediators in the TAC-induced hypertension-complicated left ventricular remodeling mouse model. The inhibition of NLRP3 expression by pirfenidone attenuated the expression of IL-1beta and IL-1beta-induced inflammatory and profibrotic responses. Pirfenidone may be useful in the treatment of hypertension-induced myocardial fibrosis by inhibiting NLRP3-induced inflammation and fibrosis.
PMID:23839341 Wang Y et al; Cardiology 126 (1): 1-11 (2013)
/EXPL THER/ Systemic sclerosis (SSc)-associated interstitial lung disease (SSc-ILD) has become the leading SSc-related cause of death. Although various types of immunosuppressive therapy have been attempted for patients with SSc-ILD, no curative or effective treatment strategies for SSc-ILD have been developed. Therefore, management of patients with SSc-ILD remains a challenge. Here, we report a Chinese, female, SSc-ILD patient who was negative for Scl-70 and showed an excellent response to pirfenidone without obvious adverse effects. She had been suffered from dry cough and exertional dyspnea for 2 months. The chest computed tomography manifestation was consistent with a pattern of fibrotic nonspecific interstitial pneumonia. The pulmonary function test showed isolated impaired diffusion. After 11 weeks of administration of pirfenidone, the dry cough and dyspnea had disappeared. Both of the lung shadows and the pulmonary diffusion function were improved. Pirfenidone might be an effective option for early SSc-ILD treatment. A well-controlled clinical trial is expected in the future.
PMID:27399114 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5058843 Huang H et al; Medicine (Baltimore) 95 (27): e4113 (2016)
Elevations in serum transaminase (ALT and/or AST) concentrations exceeding 3 times the upper limit of normal (ULN) have occurred in patients receiving pirfenidone; concomitant elevations in bilirubin concentrations have been reported rarely. ALT or AST elevations of at least 3 times the ULN were reported in 3.7% of patients receiving pirfenidone compared with 0.8% of patients receiving placebo in clinical studies; ALT or AST elevations of 10 times the ULN or greater occurred in 0.3% of pirfenidone-treated patients. Increases in liver enzymes were reversible following dosage modification or interruption of therapy. No cases of liver transplant or death due to liver failure related to pirfenidone use have been reported to date; however, the manufacturer states that the combination of transaminase elevations and hyperbilirubinemia without evidence of obstruction is generally recognized as an important predictor of severe liver injury possibly resulting in death or the need for liver transplant in some patients. Liver function tests including ALT, AST, and bilirubin concentrations should be performed prior to initiation of pirfenidone therapy, monthly for the first 6 months, and then every 3 months thereafter. Interruption of therapy and/or dosage reduction may be necessary in patients experiencing liver enzyme elevations.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2866-7
Cigarette smoking reduces peak plasma concentrations and systemic exposure to pirfenidone by 32 and 54%, respectively. The manufacturer recommends that patients be encouraged to stop smoking prior to initiation of pirfenidone and to avoid smoking during therapy.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2867
There are no adequate and well-controlled studies of Esbriet in pregnant women. Pirfenidone was not teratogenic in rats and rabbits. Because animal reproduction studies are not always predictive of human response, Esbriet should be used during pregnancy only if the benefit outweighs the risk to the patient.
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
Adverse effects reported in 10% or more of patients receiving pirfenidone and at an incidence greater than with placebo include nausea, rash, abdominal pain, upper respiratory tract infection, diarrhea, fatigue, headache, dyspepsia, dizziness, vomiting, anorexia, gastroesophageal reflux disease (GERD), sinusitis, insomnia, decreased weight, and arthralgia.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2867
For more Drug Warnings (Complete) data for Pirfenidone (16 total), please visit the HSDB record page.
For the treatment of idiopathic pulmonary fibrosis (IPF).
Esbriet is indicated in adults for the treatment of mild to moderate idiopathic pulmonary fibrosis.
Idiopathic Pulmonary Fibrosis (in adults)
Pirfenidone AET is indicated in adults for the treatment of mild to moderate idiopathic pulmonary fibrosis (IPF).
Pirfenidone is a novel agent with anti-inflammatory, antioxidant, and antifibrotic properties. It may improve lung function and reduce the number of acute exacerbations in patients with idiopathic pulmonary fibrosis (IPF).
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
L04AX05
L04AX05
L04AX05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AX - Other immunosuppressants
L04AX05 - Pirfenidone
Absorption
Rapidly absorbed following oral administration.
Volume of Distribution
The steady-state volume of distribution following oral administration is approximately 70L.
Esbriet binds to human plasma proteins, primarily to serum albumin, in a concentration-independent manner over the range of concentrations observed in clinical trials. The overall mean binding was 58% at concentrations observed in clinical studies (1 to 10 ug/mL). Mean apparent oral volume of distribution is approximately 59 to 71 liters.
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
After single oral-dose administration of 801 mg Esbriet, the maximum observed plasma concentration (Cmax) was achieved between 30 minutes and 4 hours (median time of 0.5 hours). Food decreased the rate and extent of absorption. Median Tmax increased from 0.5 hours to 3 hours with food. Maximum plasma concentrations and AUC0-inf decreased by approximately 49% and 16% with food, respectively.
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
Pirfenidone is excreted predominantly as metabolite 5-carboxy-pirfenidone, mainly in the urine (approximately 80% of the dose). The majority of Esbriet was excreted as the 5-carboxy metabolite (approximately 99.6% of that recovered).
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
/MILK/ A study with radio-labeled pirfenidone in rats has shown that pirfenidone or its metabolites are excreted in milk. It is not known whether Esbriet is excreted in human milk.
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
... This study aimed to evaluate the pharmacokinetics and urinary excretion of pirfenidone and its major metabolite 5-carboxy-pirfenidone in healthy Chinese subjects under fed conditions. 20 healthy subjects of either sex were recruited in this randomized, single-center, and open-label, single ascending doses (200, 400, and 600 mg) and multiple doses (400 mg, 3 times daily) study. Safety was assessed by adverse events, ECGs, vital signs, and clinical laboratory parameters. Blood and urine samples were analyzed with a validated LC/MS method. Pirfenidone was safe and well tolerated. After single-dose administration, pirfenidone was rapidly absorbed with a mean Tmax of 1.8-2.2 hr and a mean half life of 2.1-2.4 hr. 5-carboxy-pirfenidone was rapidly formed with a mean Tmax of 1.5-2.2 hr and a mean half life of 2.1-2.6 hr. Cmax and AUC for both parent and metabolite were dose proportional over the 200-600 mg dose range. No gender effect was found. In the steady state, the accumulation index (R) estimated for the 3 dosing intervals ranged from 1.1 to 1.5 for both pirfenidone and 5-carboxy-pirfenidone, indicating that the exposure of pirfenidone and 5-carboxy-pirfenidone increased slightly with repeated dosing, but half life and CL/F remained unchanged. Metabolism is the primary mechanism of drug clearance of pirfenidone. About 87.76% of the administered pirfenidone was excreted in urine in the form of 5-carboxy-pirfenidone, while only 0.6159% of the administered pirfenidone was detected as the unchanged form in urine.
PMID:23580109 Huang NY et al; Drug Res (Stuttg) 63 (8): 388-95 (2013)
Pirfenidone is excreted predominantly as metabolite 5-carboxy-pirfenidone, mainly in the urine (approximately 80% of the dose). The majority of Esbriet was excreted as the 5-carboxy metabolite (approximately 99.6% of that recovered).
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
In vitro profiling studies in hepatocytes and liver microsomes have shown that Esbriet is primarily metabolized in the liver by CYP1A2 and multiple other CYPs (CYP2C9, 2C19, 2D6, and 2E1). Oral administration of Esbriet results in the formation of four metabolites. In humans, only pirfenidone and 5-carboxy-pirfenidone are present in plasma in significant quantities. The mean metabolite-to-parent ratio ranged from approximately 0.6 to 0.7. No formal radiolabeled studies have assessed the metabolism of pirfenidone in humans. In vitro data suggests that metabolites are not expected to be pharmacologically active at observed metabolite concentrations.
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
2-2.5 hours
The mean terminal half-life is approximately 3 hours in healthy subjects.
NIH; DailyMed. Current Medication Information for Esbriet (Pirfenidone) Capsule (Updated: September 2015). Available from, as of July 15, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e8c3537-36d7-4de5-9b5c-7a624b9a9e6e
... This study aimed to evaluate the pharmacokinetics and urinary excretion of pirfenidone and its major metabolite 5-carboxy-pirfenidone in healthy Chinese subjects under fed conditions. 20 healthy subjects of either sex were recruited in this randomized, single-center, and open-label, single ascending doses (200, 400, and 600 mg) and multiple doses (400 mg, 3 times daily) study. ... After single-dose administration, pirfenidone was rapidly absorbed with a mean ... half life of 2.1-2.4 hr. 5-carboxy-pirfenidone was rapidly formed with ... a mean half life of 2.1-2.6 hr. ...
PMID:23580109 Huang NY et al; Drug Res (Stuttg) 63 (8): 388-95 (2013)
Although the precise mechanism of action of pirfenidone and its specific molecular targets have yet to be elucidated, the molecule has demonstrated anti-fibrotic, anti-inflammatory, and antioxidant activity. One vital anti-fibrotic mechanism involves suppression of TGF-1 (transforming growth factor-1), a key cytokine involved in fibrogenesis and extracellular matrix production. There is also evidence to suggest that pirfenidone has the ability to downregulate the expression of potent pro-inflammatory cytokines including TNF-, interleukin-1, and interferon gamma. In animal models, pirfenidone can inhibit both the influx of inflammatory cells and the increased pulmonary vascular permeability induced by bleomycin.
Dysfunctional mitochondria participate in the progression of chronic kidney disease (CKD). Pirfenidone is a newly identified anti-fibrotic drug. However, its mechanism remains unclear. Mitochondrial dysfunction is an early event that occurs prior to the onset of renal fibrosis. In this context, we investigated the protective effect of pirfenidone on mitochondria and its relevance to apoptosis and oxidative stress in renal proximal tubular cells. A remnant kidney rat model was established. Human renal proximal tubular epithelial cells (HK2) using rotenone, a mitochondrial respiratory chain complex I inhibitor were further investigated in vitro to examine the mitochondrial protective effect of pirfenidone. Pirfenidone protected mitochondrial structures and functions by stabilizing the mitochondrial membrane potential, maintaining ATP production and improving the mitochondrial DNA (mtDNA) copy number. Pirfenidone decreased tubular cell apoptosis by inhibiting the mitochondrial apoptotic signaling pathway. Pirfenidone also reduced oxidative stress by enhancing manganese superoxide dismutase (Mn-SOD) and inhibiting intracellular reactive oxygen species (ROS) generation, which suggested that the anti-oxidant effects occurred at least partially via the mitochondrial pathway. Pirfenidone may be effective prior to the onset of renal fibrosis because this drug exerts its anti-fibrotic effect by protection of mitochondria in renal proximal tubular cells.
PMID:24349535 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3857290 Chen JF et al; PLoS One 8 (12): e83593 (2013)
Pirfenidone is a drug recently approved for idiopathic pulmonary fibrosis but its mechanisms of action are partially unknown. We have previously demonstrated that the airways of patients with idiopathic pulmonary fibrosis contain procoagulant microparticles that activate coagulation factor X to its active form, Xa, a proteinase that signals fibroblast growth and differentiation, thus potentially contributing to the pathogenesis of the disease. We also reported that in vitro exposure of human alveolar cells to H2O2 causes microparticle generation. Since p38 activation is involved in microparticle generation in some cell models and p38 inhibition is one of the mechanisms of action of pirfenidone, we investigated the hypothesis that H2O2-induced generation of microparticles by alveolar cells is dependent on p38 phosphorylation and is inhibited by pirfenidone. H2O2 stimulation of alveolar cells caused p38 phosphorylation that was inhibited by pirfenidone. The drug also inhibited H2O2 induced microparticle generation as assessed by two independent methods (solid phase thrombin generation and flow cytometry). The shedding of microparticle-bound tissue factor activity was also inhibited by pirfenidone. Inhibition of p38-mediated generation of procoagulant microparticle is a previously unrecognized mechanism of action of the antifibrotic drug, pirfenidone.
PMID:27237042 Neri T et al; Pulm Pharmacol Ther. 2016 May 26. pii: S1094-5539(16)30035-9. doi: 10.1016/j.pupt.2016.05.003. (Epub ahead of print)
Recent studies indicate that pirfenidone (PFD) may have anti-fibrotic effects in many tissues, but the potential molecular mechanism remains unknown. The purpose of this study is to investigate the potential effects of PFD on epithelial-to-mesenchymal transition (EMT) and renal fibrosis in a unilateral ureteral obstruction (UUO) rat model and the involved molecular mechanism related to cultured human renal proximal tubular epithelial cells (HK-2). Sixty rats were randomly divided into three groups: sham-operated, vehicle-treated UUO, and PFD-treated UUO. Kidney specimens were collected at day 7 or 14 after UUO. PFD treatment was also performed for human HK-2. The tubulointerstitial injury, interstitial collagen deposition, and expression of type I and III collagen, a-SMA, S100A4, fibronection and E-cadherin were assessed. In addition, extracellular signal regulated kinase (ERK1/2), p38 MAPK (p38), and c-Jun N-terminal kinase/stress-activated protein kinase (JNK) were also detected. In vitro, PFD significantly attenuated TGF-beta1-induced EMT and extracellular matrix (ECM) synthesis, as determined by reducing expression of a-SMA, type I and III collagen, S100A4, fibronection, and increased expression of E-cadherin. PFD treatment attenuated TGF-beta1-induced up-regulation of phosphorylation of ERK1/2, p38 and JNK. In vivo, PFD reduced the degree of tubulointerstitial injury and renal fibrosis, which was associated with reduced expression of TGF-beta1, type III collagen, a-SMA, S100A4, fibronection, and increased expression of E-cadherin. These results suggest that pirfenidone is able to attenuate EMT and fibrosis in vivo and in vitro through antagonizing the MAPK pathway, providing a potential treatment to alleviate renal tubulointerstitial fibrosis.
PMID:27245114 Li Z et al; Nephrology (Carlton). 2016 Jun 1. doi: 10.1111/nep.12831. (Epub ahead of print)
Pirfenidone is a novel anti-fibrotic and anti-inflammatory agent that inhibits the progression of fibrosis in animal models and in patients with idiopathic pulmonary fibrosis (IPF). We previously showed that pirfenidone inhibits the over-expression of collagen type I and of heat shock protein (HSP) 47, a collagen-specific molecular chaperone, in human lung fibroblasts stimulated with transforming growth factor (TGF)-beta1 in vitro. The increased numbers of HSP47-positive type II pneumocytes as well as fibroblasts were also diminished by pirfenidone in an animal model of pulmonary fibrosis induced by bleomycin. The present study evaluates the effects of pirfenidone on collagen type I and HSP47 expression in the human alveolar epithelial cell line, A549 cells in vitro. The expression of collagen type I, HSP47 and E-cadherin mRNAs in A549 cells stimulated with TGF-beta1 was evaluated by Northern blotting or real-time PCR. The expression of collagen type I, HSP47 and fibronectin proteins was assessed by immunocytochemical staining. TGF-beta1 stimulated collagen type I and HSP47 mRNA and protein expression in A549 cells, and pirfenidone significantly inhibited this process. Pirfenidone also inhibited over-expression of the fibroblast phenotypic marker fibronectin in A549 cells induced by TGF-beta1. We concluded that the anti-fibrotic effects of pirfenidone might be mediated not only through the direct inhibition of collagen type I expression but also through the inhibition of HSP47 expression in alveolar epithelial cells, which results in reduced collagen synthesis in lung fibrosis. Furthermore, pirfenidone might partially inhibit the epithelial-mesenchymal transition.
PMID:22694981 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3403980 Hisatomi K et al; BMC Pulm Med. 2012 Jun 13;12:24. doi: 10.1186/1471-2466-12-24.
Polarized T helper type 2 (Th2) response is linked with fibrosis. Here, we evaluated the effect of the anti-fibrotic agent pirfenidone on Th type 1 (Th1) and Th2 responses. For in vivo testing; Wistar rats were made cirrhotic by intraperitoneal administration of thioacetamide. Once hepatic damage was established, pirfenidone was administered intragastrically on a daily basis during three weeks. Gene expression of Th marks was evaluated by RT-PCR and Western blot assays from liver homogenates. Pirfenidone therapy induced down-regulation of Th2 transcripts and proteins (GATA3 and IL-4), without affecting significantly Th1 genes expression (T-bet and IFN-gamma). We found that the activated form of p38 MAPK (identified by Western blot) was reduced by pirfenidone treatment, which is consistent with the anti-Th2 activity observed. Pirfenidone reduced GATA3 nuclear localization without modifying its DNA binding activity (evaluated by electrophoretic mobility shift assay). For in vitro testing; human naive CD4+ T cells were cultured in either Th1 or Th2 polarizing conditions in the presence of pirfenidone and flow cytometric analysis of intracellular synthesis of IFN-gamma and IL-4 was conducted. Pirfenidone impaired development of Th2 subpopulation. In conclusion, pirfenidone is capable of impairing Th2 differentiation and limits Th2 profibrogenic response. The mechanism involves p38 inhibition and regulation of GATA3 expression and translocation.
PMID:22222821 Navarro-Partida J et al; Eur J Pharmacol 678 (1-3): 71-7 (2012)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
81
PharmaCompass offers a list of Pirfenidone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pirfenidone manufacturer or Pirfenidone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pirfenidone manufacturer or Pirfenidone supplier.
PharmaCompass also assists you with knowing the Pirfenidone API Price utilized in the formulation of products. Pirfenidone API Price is not always fixed or binding as the Pirfenidone Price is obtained through a variety of data sources. The Pirfenidone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pirfenidone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pirfenidone, including repackagers and relabelers. The FDA regulates Pirfenidone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pirfenidone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pirfenidone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pirfenidone supplier is an individual or a company that provides Pirfenidone active pharmaceutical ingredient (API) or Pirfenidone finished formulations upon request. The Pirfenidone suppliers may include Pirfenidone API manufacturers, exporters, distributors and traders.
click here to find a list of Pirfenidone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pirfenidone DMF (Drug Master File) is a document detailing the whole manufacturing process of Pirfenidone active pharmaceutical ingredient (API) in detail. Different forms of Pirfenidone DMFs exist exist since differing nations have different regulations, such as Pirfenidone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pirfenidone DMF submitted to regulatory agencies in the US is known as a USDMF. Pirfenidone USDMF includes data on Pirfenidone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pirfenidone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pirfenidone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Pirfenidone Drug Master File in Japan (Pirfenidone JDMF) empowers Pirfenidone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Pirfenidone JDMF during the approval evaluation for pharmaceutical products. At the time of Pirfenidone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Pirfenidone suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Pirfenidone Drug Master File in Korea (Pirfenidone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Pirfenidone. The MFDS reviews the Pirfenidone KDMF as part of the drug registration process and uses the information provided in the Pirfenidone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Pirfenidone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Pirfenidone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Pirfenidone suppliers with KDMF on PharmaCompass.
A Pirfenidone CEP of the European Pharmacopoeia monograph is often referred to as a Pirfenidone Certificate of Suitability (COS). The purpose of a Pirfenidone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Pirfenidone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Pirfenidone to their clients by showing that a Pirfenidone CEP has been issued for it. The manufacturer submits a Pirfenidone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Pirfenidone CEP holder for the record. Additionally, the data presented in the Pirfenidone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Pirfenidone DMF.
A Pirfenidone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Pirfenidone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Pirfenidone suppliers with CEP (COS) on PharmaCompass.
A Pirfenidone written confirmation (Pirfenidone WC) is an official document issued by a regulatory agency to a Pirfenidone manufacturer, verifying that the manufacturing facility of a Pirfenidone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Pirfenidone APIs or Pirfenidone finished pharmaceutical products to another nation, regulatory agencies frequently require a Pirfenidone WC (written confirmation) as part of the regulatory process.
click here to find a list of Pirfenidone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pirfenidone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pirfenidone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pirfenidone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pirfenidone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pirfenidone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pirfenidone suppliers with NDC on PharmaCompass.
Pirfenidone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pirfenidone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pirfenidone GMP manufacturer or Pirfenidone GMP API supplier for your needs.
A Pirfenidone CoA (Certificate of Analysis) is a formal document that attests to Pirfenidone's compliance with Pirfenidone specifications and serves as a tool for batch-level quality control.
Pirfenidone CoA mostly includes findings from lab analyses of a specific batch. For each Pirfenidone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pirfenidone may be tested according to a variety of international standards, such as European Pharmacopoeia (Pirfenidone EP), Pirfenidone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pirfenidone USP).

