
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
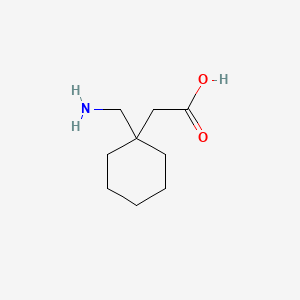

1. 1-(aminomethyl)cyclohexaneacetic Acid
2. Apo Gabapentin
3. Apo-gabapentin
4. Apogabapentin
5. Convalis
6. Gabapentin Hexal
7. Gabapentin Ratiopharm
8. Gabapentin Stada
9. Gabapentin-ratiopharm
10. Neurontin
11. Novo Gabapentin
12. Novo-gabapentin
13. Novogabapentin
14. Pms-gabapentin
1. 60142-96-3
2. Neurontin
3. 1-(aminomethyl)cyclohexaneacetic Acid
4. Gabapentine
5. Aclonium
6. 2-[1-(aminomethyl)cyclohexyl]acetic Acid
7. Gabapetin
8. Gabapentinum
9. 2-(1-(aminomethyl)cyclohexyl)acetic Acid
10. Cyclohexaneacetic Acid, 1-(aminomethyl)-
11. Fanatrex
12. Gabapentino
13. Gralise
14. Serada
15. [1-(aminomethyl)cyclohexyl]acetic Acid
16. (1-aminomethyl-cyclohexyl)-acetic Acid
17. Goe-3450
18. Gabapentina
19. Go 3450
20. Ci-945
21. C9h17no2
22. Dm-1796
23. Goe 2450
24. Goe 3450
25. Ci 945
26. 1-(aminomethyl)-cyclohexaneacetic Acid
27. Gabarone
28. Dm-5689
29. Chembl940
30. Nsc-742194
31. Gabapentin; Neurontin
32. 6cw7f3g59x
33. Chebi:42797
34. Gabapentino [spanish]
35. Nsc-759254
36. Gabapentine [inn-french]
37. Gabapentinum [inn-latin]
38. Ncgc00015466-08
39. Gabapen
40. Dsstox_cid_74
41. Gabapentino [inn-spanish]
42. Vultin
43. Cas-60142-96-3
44. Gabapentin Gr
45. Dsstox_rid_75350
46. Dsstox_gsid_20074
47. Gbn
48. Gabapentinium
49. Gabapentin (neurontin)
50. Neuontin
51. Sefelsa
52. Therapentin-90
53. Neurontin (tn)
54. Ccris 7210
55. Hsdb 7364
56. Sr-01000000019
57. Einecs 262-076-3
58. Mfcd00865286
59. Brn 2359739
60. Unii-6cw7f3g59x
61. Neurentin
62. Gabapentin-er
63. Novo-gabapentine
64. Gabapentin, Solid
65. Gabapentin [usan:usp:inn:ban]
66. Gabapentin Solution
67. Dds-2003
68. Prestwick_151
69. 1-(aminomethyl)cyclohexaneaceticacid
70. Gralise (tn)
71. Carbatin
72. Gabapetine
73. Tocris-0806
74. Gabapentin [mi]
75. Lopac-g-154
76. Gabapentin [inn]
77. Gabapentin [jan]
78. Prestwick0_000861
79. Prestwick1_000861
80. Prestwick2_000861
81. Prestwick3_000861
82. Gabapentin [hsdb]
83. Gabapentin [usan]
84. G-154
85. Gabapentin [vandf]
86. Gabapentin [mart.]
87. Schembl8343
88. Gabapentin [usp-rs]
89. Gabapentin [who-dd]
90. Lopac0_000582
91. Bspbio_000901
92. Gabapentin (jan/usp/inn)
93. Mls000069358
94. Bidd:gt0656
95. Spbio_002822
96. Bpbio1_000993
97. Gtpl5483
98. Zinc4949
99. Dtxsid0020074
100. Gabapentin [ep Impurity]
101. Gabapentin [orange Book]
102. Gabapentin [ep Monograph]
103. Gabapentin [usp Monograph]
104. Hms1570n03
105. Hms2089j03
106. Hms2097n03
107. Hms2236o03
108. Hms3261f06
109. Hms3650a20
110. Hms3714n03
111. 1-aminomethyl Cyclohexaneacetic Acid
112. 1-aminomethylcyclohexane Acetic Acid
113. Act03340
114. Bcp25698
115. Hy-a0057
116. 1-(aminomethyl)cyclohexanacetic Acid
117. Tox21_110157
118. Tox21_500582
119. Bbl010794
120. Bdbm50080153
121. Nsc742194
122. S2133
123. Stk598009
124. 1-(aminomethyl)cyclohexyl-acetic Acid
125. 1-amino Methyl Cyclohexane-acetic Acid
126. Akos000280865
127. Tox21_110157_1
128. 1-aminomethyl-1cyclohexane-acetic Acid
129. Ab07485
130. Ac-1485
131. Ccg-204671
132. Cs-1545
133. Db00996
134. Ks-1064
135. Lp00582
136. Nsc 742194
137. Nsc 759254
138. Sdccgsbi-0050564.p002
139. 1-aminomethyl-1-cyclohexane-acetic Acid
140. Ncgc00015466-01
141. Ncgc00015466-02
142. Ncgc00015466-03
143. Ncgc00015466-04
144. Ncgc00015466-05
145. Ncgc00015466-06
146. Ncgc00015466-07
147. Ncgc00015466-09
148. Ncgc00015466-11
149. Ncgc00015466-27
150. Ncgc00016891-01
151. Ncgc00021545-02
152. Ncgc00021545-04
153. Ncgc00021545-05
154. Ncgc00261267-01
155. Smr000058311
156. Sbi-0206904.p001
157. Am20070538
158. Eu-0100582
159. Ft-0626586
160. Ft-0668920
161. G0318
162. En300-52516
163. 42g963
164. C07018
165. D00332
166. Gabapentin 100 Microg/ml In Acetonitrile:methanol
167. L000733
168. Q410352
169. Sr-01000000019-2
170. Sr-01000000019-6
171. Sr-01000000019-11
172. Z1258578343
173. Gabapentin, European Pharmacopoeia (ep) Reference Standard
174. Gabapentin, United States Pharmacopeia (usp) Reference Standard
175. Gabapentin, Pharmaceutical Secondary Standard; Certified Reference Material
176. Gabapentin Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
177. Gabapentin Solution, 10 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 171.24 g/mol |
|---|---|
| Molecular Formula | C9H17NO2 |
| XLogP3 | -1.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 171.125928785 g/mol |
| Monoisotopic Mass | 171.125928785 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 162 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Gabapentin |
| PubMed Health | Gabapentin (By mouth) |
| Drug Classes | Anticonvulsant, Neuropathic Pain Agent |
| Drug Label | Gabapentin tablets USP are an anticonvulsant for oral administration. Gabapentin USP is described as 1-(aminomethyl)cyclohexaneacetic acid. Gabapentin USP is a white to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely s... |
| Active Ingredient | Gabapentin |
| Dosage Form | Tablet; Capsule; Solution |
| Route | oral; Oral |
| Strength | 600mg; 250mg/5ml; 800mg; 400mg; 300mg; 100mg |
| Market Status | Tentative Approval; Prescription |
| Company | Amneal Pharms; Mylan Pharms; Ranbaxy; Marksans Pharma; Apotex; Alkem; Aurobindo Pharma; Sun Pharm Inds; Taro; Invagen Pharms; Hikma Pharms; Hikma; Amneal Pharms Ny; Glenmark Generics; Ivax Sub Teva Pharms; Actavis Elizabeth; Teva Pharms; Hi Tech Pharma; A |
| 2 of 6 | |
|---|---|
| Drug Name | Gralise |
| PubMed Health | Gabapentin (By mouth) |
| Drug Classes | Anticonvulsant, Neuropathic Pain Agent |
| Drug Label | Gabapentin is 1-(aminomethyl)cyclohexaneacetic acid; -amino-2-cyclohexyl-butyric acid with a molecular formula of C9H17NO2 and a molecular weight of 171.24.The structural formula is:Gabapentin is a white to off-white crystalline solid with a pKa1 o... |
| Active Ingredient | Gabapentin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg; 300mg |
| Market Status | Prescription |
| Company | Depomed |
| 3 of 6 | |
|---|---|
| Drug Name | Neurontin |
| Drug Label | Neurontin (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containi... |
| Active Ingredient | Gabapentin |
| Dosage Form | Tablet; Capsule; Solution |
| Route | oral; Oral |
| Strength | 600mg; 250mg/5ml; 800mg; 400mg; 300mg; 100mg; 0.0 |
| Market Status | Prescription |
| Company | Pfizer Pharms; Parke Davis |
| 4 of 6 | |
|---|---|
| Drug Name | Gabapentin |
| PubMed Health | Gabapentin (By mouth) |
| Drug Classes | Anticonvulsant, Neuropathic Pain Agent |
| Drug Label | Gabapentin tablets USP are an anticonvulsant for oral administration. Gabapentin USP is described as 1-(aminomethyl)cyclohexaneacetic acid. Gabapentin USP is a white to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely s... |
| Active Ingredient | Gabapentin |
| Dosage Form | Tablet; Capsule; Solution |
| Route | oral; Oral |
| Strength | 600mg; 250mg/5ml; 800mg; 400mg; 300mg; 100mg |
| Market Status | Tentative Approval; Prescription |
| Company | Amneal Pharms; Mylan Pharms; Ranbaxy; Marksans Pharma; Apotex; Alkem; Aurobindo Pharma; Sun Pharm Inds; Taro; Invagen Pharms; Hikma Pharms; Hikma; Amneal Pharms Ny; Glenmark Generics; Ivax Sub Teva Pharms; Actavis Elizabeth; Teva Pharms; Hi Tech Pharma; A |
| 5 of 6 | |
|---|---|
| Drug Name | Gralise |
| PubMed Health | Gabapentin (By mouth) |
| Drug Classes | Anticonvulsant, Neuropathic Pain Agent |
| Drug Label | Gabapentin is 1-(aminomethyl)cyclohexaneacetic acid; -amino-2-cyclohexyl-butyric acid with a molecular formula of C9H17NO2 and a molecular weight of 171.24.The structural formula is:Gabapentin is a white to off-white crystalline solid with a pKa1 o... |
| Active Ingredient | Gabapentin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 600mg; 300mg |
| Market Status | Prescription |
| Company | Depomed |
| 6 of 6 | |
|---|---|
| Drug Name | Neurontin |
| Drug Label | Neurontin (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containi... |
| Active Ingredient | Gabapentin |
| Dosage Form | Tablet; Capsule; Solution |
| Route | oral; Oral |
| Strength | 600mg; 250mg/5ml; 800mg; 400mg; 300mg; 100mg; 0.0 |
| Market Status | Prescription |
| Company | Pfizer Pharms; Parke Davis |
Analgesics; Anti-Anxiety Agents; Anticonvulsants; Antimanic Agents; Antiparkinson Agents; Calcium Channel Blockers; Excitatory Amino Acid Antagonists
National Library of Medicine's Medical Subject Headings. Gabapentin. Online file (MeSH, 2016). Available from, as of October 28, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
Neurontin is indicated for: Management of postherpetic neuralgia in adults. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Neurontin (Gabapentin) Capsule; Neurontin (Gabapentin) Tablet, Film-coated; Neurontin (Gabapentin) Solution (Updated: August 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388
Neurontin is indicated for: Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Neurontin (Gabapentin) Capsule; Neurontin (Gabapentin) Tablet, Film-coated; Neurontin (Gabapentin) Solution (Updated: August 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388
Horizant (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. /Included in US product label/
NIH; DailyMed. Current Medication Information for Horizant (Gabapentin Enacarbil) Extended-Release Tablets (Updated: July 2015). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c486fc7-c8c4-4c6c-b30c-366dabaeaadd
For more Therapeutic Uses (Complete) data for GABAPENTIN (12 total), please visit the HSDB record page.
VET: In general, avoid the use of the commercially available human oral solution (Neurontin) in dogs as it reportedly contains 300 mg/mL xylitol. As the threshold dose that can cause hypoglycemia in dogs is approximately 100 mg/kg doses of up to 15 mg/kg in dogs using the solution should be safe, but further data is needed to confirm this Additionally, xylitol may be hepatotoxic in dogs. Doses of 500 mg/kg of xylitol are thought to be the threshold for this toxicity, but there have been anecdotal reports of it occuring at much lower doss. In cats, at the dosages used presently, xylitol toxicity dosen not appear to be a problem with gabapentin oral solcuiton, but sue with caution.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 649
VET: Sedation and ataxia are probably the most likely adverse effects seen in small animals. Starting the dose at the lower end of the range and increasing with time may alleviate these effects.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 650
Gabapentin and gabapentin enacarbil should be used during pregnancy only when the potential benefits justify the possible risks to the fetus.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2326
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
NIH; DailyMed. Current Medication Information for Neurontin (Gabapentin) Capsule; Neurontin (Gabapentin) Tablet, Film-coated; Neurontin (Gabapentin) Solution (Updated: August 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388
For more Drug Warnings (Complete) data for GABAPENTIN (37 total), please visit the HSDB record page.
In the United States, gabapentin is officially indicated for the treatment of postherpetic neuralgia in adults and for the adjunctive treatment of partial-onset seizures, with or without secondary generalization, in patients 3 years of age and older. In Europe, gabapentin is indicated for adjunctive therapy in the treatment of partial-onset seizures, with or without secondary generalization, in patients 6 years of age and older and as monotherapy in patients 12 years of age and older. It is also used in adults for the treatment of various types of peripheral neuropathic pain, such as painful diabetic neuropathy.
Treatment of chronic pain
Treatment of postherpetic neuralgia
Gabapentin is an anti-convulsant medication that inhibits the release of excitatory neurotransmitters, allowing for its use against pathologic neurotransmission such as that seen in neuropathic pain and seizure disorders. It has a wide therapeutic index, with doses in excess of 8000 mg/kg failing to cause a fatal reaction in rats. Gabapentin is ineffective in absence seizures and should be used in caution in patients with mixed seizure disorders involving absence seizures. Gabapentin has been associated with drug reaction with eosinophilia and systemic symptoms (DRESS), otherwise known as multi-organ hypersensitivity. This reaction can prove fatal and early symptoms such as fever, lymphadenopathy, and rash should be promptly investigated.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Excitatory Amino Acid Antagonists
Drugs that bind to but do not activate excitatory amino acid receptors, thereby blocking the actions of agonists. (See all compounds classified as Excitatory Amino Acid Antagonists.)
Antimanic Agents
Agents that are used to treat bipolar disorders or mania associated with other affective disorders. (See all compounds classified as Antimanic Agents.)
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N03AX12
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N03AX12
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX12 - Gabapentin
Absorption
Absorption of gabapentin is thought to occur solely via facilitated transport by the LAT1 transporter within the intestines. As this process is saturable, the oral bioavailability of gabapentin is inversely proportional to the administered dose - the oral bioavailability of a 900mg/day regimen is approximately 60%, whereas a 4800mg/day regimen results in only 27% bioavailability. The Tmax of gabapentin has been estimated to be 2-3 hours. Food has no appreciable effect on gabapentin absorption.
Route of Elimination
Gabapentin is eliminated solely in the urine as unchanged drug. Cimetidine, an inhibitor of renal tubular secretion, reduces clearance by approximately 12%, suggesting that some degree of tubular secretion is involved in the renal elimination of gabapentin.
Volume of Distribution
The apparent volume of distribution of gabapentin after IV administration is 586 L. The drug is found in the CSF in concentrations approximately 9-20% of the corresponding plasma concentrations and is secreted into breast milk in concentrations similar to that seen in plasma.
Clearance
Both the plasma clearance and renal clearance of gabapentin are directly proportional to the patient's creatinine clearance due to its primarily renal elimination.
/MILK/ Gabapentin enters maternal milk. It has been calculated that a nursing human infant could be exposed to a maximum dosage of 1 mg/kg/day. This is 5-10% of the usual pediatric (>3 years old) therapeutic dose. In veterinary patients, this appears unlikely to be of significant clinical concern.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 650
The pharmacokinetic properties of gabapentin vary based on the specific formulation of the drug. Following oral administration, gabapentin is absorbed principally in the proximal small intestine via a saturable L-amino acid transport system; as a result, the bioavailability of the drug decreases with increasing doses. Gabapentin gastroretentive tablets are specifically formulated to swell upon contact with gastric fluid to a size that promotes gastric retention for approximately 8-10 hours when taken with a meal; this allows for gradual and slow release of the drug to the proximal small intestine, its principal site of absorption. Following administration of gabapentin gastroretentive tablets in healthy individuals, time to peak plasma concentrations of the drug was increased (about 4-6 hours longer), peak plasma concentrations were increased, and systemic exposure was decreased relative to conventional (immediate-release) gabapentin. Gabapentin enacarbil, a prodrug of gabapentin, is rapidly and efficiently converted to gabapentin by first-pass hydrolysis following oral administration. Unlike gabapentin, gabapentin enacarbil is absorbed via high-capacity transporters throughout the GI tract and is not affected by saturable absorption; this improves bioavailability of the drug and allows for dose-proportional exposure. Food has only a minimal effect on the pharmacokinetics of conventional (immediate-release) formulations of gabapentin, but increases the bioavailability of gabapentin gastroretentive tablets. Administration of gabapentin enacarbil extended-release tablets with food also increases systemic exposure of the drug compared with exposure under fasted conditions.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2326
Less than 3% of gabapentin circulates bound to plasma protein. The apparent volume of distribution of gabapentin after 150 mg intravenous administration is 58 +/- 6 L (mean +/- SD). In patients with epilepsy, steady-state predose (Cmin) concentrations of gabapentin in cerebrospinal fluid were approximately 20% of the corresponding plasma concentrations.
NIH; DailyMed. Current Medication Information for Neurontin (Gabapentin) Capsule; Neurontin (Gabapentin) Tablet, Film-coated; Neurontin (Gabapentin) Solution (Updated: August 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388
Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. ... Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. In elderly patients, and in patients with impaired renal function, gabapentin plasma clearance is reduced. Gabapentin can be removed from plasma by hemodialysis.
NIH; DailyMed. Current Medication Information for Neurontin (Gabapentin) Capsule; Neurontin (Gabapentin) Tablet, Film-coated; Neurontin (Gabapentin) Solution (Updated: August 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388
For more Absorption, Distribution and Excretion (Complete) data for GABAPENTIN (9 total), please visit the HSDB record page.
Gabapentin is not appreciably metabolized in humans - in humans, metabolites account for less than 1% of an administered dose, with the remainder being excreted as unchanged parent drug in the urine.
Elimination is primarily via renal routes, but gabapentin is partially metabolized bo N-methyl-gabapentin in dogs.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 649
All pharmacological actions following gabapentin administration are due to the activity of the parent compound; gabapentin is not appreciably metabolized in humans.
NIH; DailyMed. Current Medication Information for Neurontin (Gabapentin) Capsule; Neurontin (Gabapentin) Tablet, Film-coated; Neurontin (Gabapentin) Solution (Updated: August 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388
The elimination t1/2 of gabapentin in patients with normal renal function is 5-7 hours. In patients with reduced renal function, the elimination t1/2 may be prolonged - in patients with a creatinine clearance of <30 mL/min, the reported half-life of gabapentin was approximately 52 hours.
In dogs ... elimination half life is approximately 2-4 hours.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 649
Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing.
NIH; DailyMed. Current Medication Information for Neurontin (Gabapentin) Capsule; Neurontin (Gabapentin) Tablet, Film-coated; Neurontin (Gabapentin) Solution (Updated: August 2016). Available from, as of October 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee9ad9ed-6d9f-4ee1-9d7f-cfad438df388
In cats ... elimination half life of 2.8 hours is similar to dogs.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 649
The precise mechanism through which gabapentin exerts its therapeutic effects is unclear. The primary mode of action appears to be at the auxillary 2-1 subunit of voltage-gated calcium channels (though a low affinity for the 2-2 subunit has also been reported). The major function of these subunits is to facilitate the movement of pore-forming 1 subunits of calcium channels from the endoplasmic reticulum to the cell membrane of pre-synaptic neurons. There is evidence that chronic pain states can cause an increase in the expression of 2 subunits and that these changes correlate with hyperalgesia. Gabapentin appears to inhibit the action of 2-1 subunits, thus decreasing the density of pre-synaptic voltage-gated calcium channels and subsequent release of excitatory neurotransmitters. It is likely that this inhibition is also responsible for the anti-epileptic action of gabapentin. There is some evidence that gabapentin also acts on adenosine receptors and voltage-gated potassium channels, though the clinical relevance of its action at these sites is unclear.
Although the exact mechanism by which gabapentin exerts its analgesic effects is not known, the drug has been shown to prevent allodynia (pain-related behavior in response to normally innocuous stimuli) and hyperalgesia (exaggerated response to painful stimuli) in several models of neuropathic pain. Gabapentin also has been shown to decrease pain-related responses after peripheral inflammation in animals; however, the drug has not altered immediate pain-related behaviors. The clinical relevance of these findings is not known. In vitro studies demonstrate that gabapentin binds to the alpha2delta subunit of voltage-activated calcium channels; however, the clinical importance of this effect is not known.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2326
Gabapentin is an anticonvulsant agent structurally related to the inhibitory CNS neurotransmitter gamma-aminobutyric acid (GABA). Gabapentin enacarbil is a prodrug of gabapentin that is rapidly converted to gabapentin following oral administration; the therapeutic effects of gabapentin enacarbil are attributed to gabapentin. Although gabapentin was developed as a structural analog of GABA that would penetrate the blood-brain barrier (unlike GABA) and mimic the action of GABA at inhibitory neuronal synapses, the drug has no direct GABA-mimetic action and its precise mechanism of action has not been elucidated.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2326
Results of some studies in animals indicate that gabapentin protects against seizure and/or tonic extensions induced by the GABA antagonists picrotoxin and bicuculline or by GABA synthesis inhibitors (e.g., 3-mercaptopropionic acid, isonicotinic acid, semicarbazide). However, gabapentin does not appear to bind to GABA receptors nor affect GABA reuptake or metabolism and does not act as a precursor of GABA or of other substances active at GABA receptors. Gabapentin also has no affinity for binding sites on common neuroreceptors (e.g., benzodiazepine; glutamate; quisqualate; kainate; strychnine-insensitive or -sensitive glycine; alpha1-, alpha2-, or beta-adrenergic; adenosine A1 or A2; cholinergic [muscarinic or nicotinic]; dopamine D1 or D2; histamine H1; type 1 or 2 serotonergic [5-HT1 or 5-HT2]; opiate mc, delta, or k) or ion channels (e.g., voltage-sensitive calcium channel sites labeled with nitrendipine or diltiazem, voltage-sensitive sodium channel sites labeled with batrachotoxinin A 20alpha-benzoate). Conflicting results have been reported in studies of gabapentin affinity for and activity at N-methyl-d-aspartic acid (NMDA) receptors.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2326
Currently, the clinical management of visceral pain remains unsatisfactory for many patients suffering from this disease. While preliminary animal studies have suggested the effectiveness of gabapentin in successfully treating visceral pain, the mechanism underlying its analgesic effect remains unclear. Evidence from other studies has demonstrated the involvement of protein kinase C (PKC) and extracellular signal-regulated kinase1/2 (ERK1/2) in the pathogenesis of visceral inflammatory pain. In this study, we tested the hypothesis that gabapentin produces analgesia for visceral inflammatory pain through its inhibitory effect on the PKC-ERK1/2 signaling pathway. Intracolonic injections of formalin were performed in rats to produce colitis pain. Our results showed that visceral pain behaviors in these rats decreased after intraperitoneal injection of gabapentin. These behaviors were also reduced by intrathecal injections of the PKC inhibitor, H-7, and the ERK1/2 inhibitor, PD98059. Neuronal firing of wide dynamic range neurons in L6-S1 of the rat spinal cord dorsal horn were significantly increased after intracolonic injection of formalin. This increased firing rate was inhibited by intraperitoneal injection of gabapentin and both the individual and combined intrathecal application of H-7 and PD98059. Western blot analysis also revealed that PKC membrane translocation and ERK1/2 phosphorylation increased significantly following formalin injection, confirming the recruitment of PKC and ERK1/2 during visceral inflammatory pain. These effects were also significantly reduced by intraperitoneal injection of gabapentin. Therefore, we concluded that the analgesic effect of gabapentin on visceral inflammatory pain is mediated through suppression of PKC and ERK1/2 signaling pathways. Furthermore, we found that the PKC inhibitor, H-7, significantly diminished ERK1/2 phosphorylation levels, implicating the involvement of PKC and ERK1/2 in the same signaling pathway. Thus, our results suggest a novel mechanism of gabapentin-mediated analgesia for visceral inflammatory pain through a PKC-ERK1/2 signaling pathway that may be a future therapeutic target for the treatment of visceral inflammatory pain.
PMID:26512901 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4626203 Zhang YB et al; PLoS One. 2015 Oct 29;10(10):e0141142. doi: 10.1371/journal.pone.0141142. eCollection 2015.
The gabapentinoids (pregabalin and gabapentin) are first line treatments for neuropathic pain. They exert their actions by binding to the alpha2delta (a2d) accessory subunits of voltage-gated Ca2+ channels. Because these subunits interact with critical aspects of the neurotransmitter release process, gabapentinoid binding prevents transmission in nociceptive pathways. Gabapentinoids also reduce plasma membrane expression of voltage-gated Ca2+ channels but this may have little direct bearing on their therapeutic actions. In animal models of neuropathic pain, gabapentinoids exert an anti-allodynic action within 30 minutes but most of their in vitro effects are 30-fold slower, taking at least 17 hours to develop. This difference may relate to increased levels of a2d expression in the injured nervous system. Thus, in situations where a2d is experimentally upregulated in vitro, gabapentinoids act within minutes to interrupt trafficking of a2d subunits to the plasma membrane within nerve terminals. When a2d is not up-regulated, gabapentinoids act slowly to interrupt trafficking of a2d protein from cell bodies to nerve terminals. This improved understanding of the mechanism of gabapentinoid action is related to their slowly developing actions in neuropathic pain patients, to the concept that different processes underlie the onset and maintenance of neuropathic pain and to the use of gabapentinoids in management of postsurgical pain.
PMID:27118808 Alles SR, Smith PA; Neuroscientist. 2016 Apr 26. pii: 1073858416628793. (Epub ahead of print)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
84
PharmaCompass offers a list of Gabapentin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Gabapentin manufacturer or Gabapentin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Gabapentin manufacturer or Gabapentin supplier.
PharmaCompass also assists you with knowing the Gabapentin API Price utilized in the formulation of products. Gabapentin API Price is not always fixed or binding as the Gabapentin Price is obtained through a variety of data sources. The Gabapentin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Gabapentin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Gabapentin, including repackagers and relabelers. The FDA regulates Gabapentin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Gabapentin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Gabapentin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Gabapentin supplier is an individual or a company that provides Gabapentin active pharmaceutical ingredient (API) or Gabapentin finished formulations upon request. The Gabapentin suppliers may include Gabapentin API manufacturers, exporters, distributors and traders.
click here to find a list of Gabapentin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Gabapentin DMF (Drug Master File) is a document detailing the whole manufacturing process of Gabapentin active pharmaceutical ingredient (API) in detail. Different forms of Gabapentin DMFs exist exist since differing nations have different regulations, such as Gabapentin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Gabapentin DMF submitted to regulatory agencies in the US is known as a USDMF. Gabapentin USDMF includes data on Gabapentin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Gabapentin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Gabapentin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Gabapentin Drug Master File in Japan (Gabapentin JDMF) empowers Gabapentin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Gabapentin JDMF during the approval evaluation for pharmaceutical products. At the time of Gabapentin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Gabapentin suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Gabapentin Drug Master File in Korea (Gabapentin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Gabapentin. The MFDS reviews the Gabapentin KDMF as part of the drug registration process and uses the information provided in the Gabapentin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Gabapentin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Gabapentin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Gabapentin suppliers with KDMF on PharmaCompass.
A Gabapentin CEP of the European Pharmacopoeia monograph is often referred to as a Gabapentin Certificate of Suitability (COS). The purpose of a Gabapentin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Gabapentin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Gabapentin to their clients by showing that a Gabapentin CEP has been issued for it. The manufacturer submits a Gabapentin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Gabapentin CEP holder for the record. Additionally, the data presented in the Gabapentin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Gabapentin DMF.
A Gabapentin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Gabapentin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Gabapentin suppliers with CEP (COS) on PharmaCompass.
A Gabapentin written confirmation (Gabapentin WC) is an official document issued by a regulatory agency to a Gabapentin manufacturer, verifying that the manufacturing facility of a Gabapentin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Gabapentin APIs or Gabapentin finished pharmaceutical products to another nation, regulatory agencies frequently require a Gabapentin WC (written confirmation) as part of the regulatory process.
click here to find a list of Gabapentin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Gabapentin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Gabapentin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Gabapentin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Gabapentin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Gabapentin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Gabapentin suppliers with NDC on PharmaCompass.
Gabapentin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Gabapentin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Gabapentin GMP manufacturer or Gabapentin GMP API supplier for your needs.
A Gabapentin CoA (Certificate of Analysis) is a formal document that attests to Gabapentin's compliance with Gabapentin specifications and serves as a tool for batch-level quality control.
Gabapentin CoA mostly includes findings from lab analyses of a specific batch. For each Gabapentin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Gabapentin may be tested according to a variety of international standards, such as European Pharmacopoeia (Gabapentin EP), Gabapentin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Gabapentin USP).

