
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Regulatory FDF Prices
NA
0
FDF
0
Weekly News Recap #Phispers
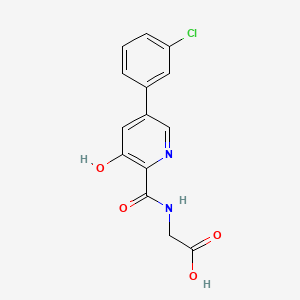

1. Akb-6548
1. 1000025-07-9
2. Akb-6548
3. Pg-1016548
4. Vadadustat [usan]
5. N-(5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl)glycine
6. B506
7. Pg1016548
8. B-506
9. Akb6548
10. (5-(3-chlorophenyl)-3-hydroxypicolinoyl)glycine
11. I60w9520vv
12. 2-[[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino]acetic Acid
13. Glycine, N-((5-(3-chlorophenyl)-3-hydroxy-2-pyridinyl)carbonyl)-
14. Glycine, N-[[5-(3-chlorophenyl)-3-hydroxy-2-pyridinyl]carbonyl]-
15. Vafseo
16. Glycine, N-[[5-(3-chlorophenyl)-3-hydroxy-2-pyridinyl]carbonyl]-;glycine, N-[[5-(3-chlorophenyl)-3-hydroxy-2-pyridinyl]carbonyl]-
17. Unii-i60w9520vv
18. Us8722895, 11: {[5-(3-chlorophenyl)-3-hydroxypyridine-2- Carbonyl]amino}-acetic Acid
19. A1z
20. Vafseo (tn)
21. Vadadustat [inn]
22. Vadadustat [jan]
23. Us8722895, 10: {[5-(3-chlorophenyl)-3-hydroxypyridine-2- Carbonyl]amino}-acetic Acid Trifluoroacetic Acid Salt
24. Vadadustat [who-dd]
25. Vadadustat (jan/usan/inn)
26. Schembl1920738
27. Chembl3646221
28. Gtpl11831
29. Bdbm107704
30. Dtxsid501179936
31. Amy27885
32. Bcp19497
33. Ex-a2573
34. 2-{[5-(3-chlorophenyl)-3-hydroxypyridin-2-yl]formamido}acetic Acid
35. Gsk128863
36. S6490
37. Zinc117532869
38. Cs-6373
39. Db12255
40. Sb19204
41. Us8598210, Table Xv, 10
42. Us8598210, Table Xv, 11
43. Ac-30928
44. As-71695
45. Db-102455
46. Hy-101277
47. Vadadustat; Pg-1016548; Akb-6548
48. J3.560.572j
49. C71001
50. D11078
51. Us8598210, 119
52. Q27280485
53. 2-(5-(3-chlorophenyl)-3-hydroxypicolinamido)acetic Acid
54. Akb-6548; B-506; Pg-1016548
55. {[5-(3-chlorophenyl)-3-hydroxy-pyridine-2-carbonyl]-amino}-acetic Acid
56. {[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino}-acetic Acid
| Molecular Weight | 306.70 g/mol |
|---|---|
| Molecular Formula | C14H11ClN2O4 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 306.0407345 g/mol |
| Monoisotopic Mass | 306.0407345 g/mol |
| Topological Polar Surface Area | 99.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 393 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of anaemia due to chronic disorders
B - Blood and blood forming organs
B03 - Antianemic preparations
B03X - Other antianemic preparations
B03XA - Other antianemic preparations
B03XA08 - Vadadustat
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
About the Company : Ami Lifesciences, established in 2006, is one of the fastest growing API manufacturing companies in India. Specializing in cardiovascular, anti-diabetic, CNS, and respiratory thera...
About the Company : HRV Pharma is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
 Maithri Drugs: Delivering Trusted, High-Quality APIs to 35+ Countries with Innovation, Compliance, and Excellence.
Maithri Drugs: Delivering Trusted, High-Quality APIs to 35+ Countries with Innovation, Compliance, and Excellence.
About the Company : Maithri Drugs is one of India's fastest growing pharmaceutical companies, strategically focused on Active Pharmaceutical Ingredients (APIs). Backed by a strong R&D foundation, we h...
About the Company : Shanvr Life Sciences Pvt Ltd is a pharmaceutical product development company with a strong emphasis on research. Our approach centers around the creation of specialized generic, on...

About the Company : In November 2020, Viatris was formed through the combination of Mylan and Upjohn, with a mission of empowering people worldwide to live healthier at every stage of life. Viatris (N...

About the Company : Zydus is headquartered in Ahmedabad, India, and ranks 4th in the Indian pharmaceutical industry. The group has manufacturing sites and research facilities spread across five states...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Undisclosed
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 30, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Undisclosed
Product Name : Undisclosed
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
March 30, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The proceeds from the offering will be used for R&D and commercialization of Vafseo (vadadustat) which is approved for the treatment of anemia due to chronic kidney disease in adults.
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Vafseo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Leerink Partners
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Public Offering March 19, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Leerink Partners
Deal Size : Undisclosed
Deal Type : Public Offering
Akebia Therapeutics Announces Proposed Public Offering of Common Stock
Details : The proceeds from the offering will be used for R&D and commercialization of Vafseo (vadadustat) which is approved for the treatment of anemia due to chronic kidney disease in adults.
Product Name : Vafseo
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
March 19, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The proceeds from the offering will be used for R&D and commercialization of Vafseo (vadadustat) which is approved for the treatment of anemia due to chronic kidney disease in adults.
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Vafseo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Leerink Partners
Deal Size: $50.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering March 19, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Leerink Partners
Deal Size : $50.0 million
Deal Type : Public Offering
Akebia Therapeutics Announces Pricing of Public Offering of Common Stock
Details : The proceeds from the offering will be used for R&D and commercialization of Vafseo (vadadustat) which is approved for the treatment of anemia due to chronic kidney disease in adults.
Product Name : Vafseo
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
March 19, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the agreement, Er-Kim partner with the MEDICE to commercialize the Vafseo (vadadustat). It is being indicated for the treatment of of symptomatic anaemia associated with CKD in adults.
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Vafseo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: MEDICE Health Family
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement January 14, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : MEDICE Health Family
Deal Size : Undisclosed
Deal Type : Agreement
Er-Kim Announces Distribution Agreement with MEDICE for Vafseo® in Europe
Details : Under the agreement, Er-Kim partner with the MEDICE to commercialize the Vafseo (vadadustat). It is being indicated for the treatment of of symptomatic anaemia associated with CKD in adults.
Product Name : Vafseo
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
January 14, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vafseo (vadadustat) is a once-daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor. It is approved for the treatment of anemia due to chronic kidney disease in adult patients on dialysis.
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Vafseo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
U.S. Renal Care Begins VOICE Trial for Vafseo® in CKD Dialysis Patients
Details : Vafseo (vadadustat) is a once-daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor. It is approved for the treatment of anemia due to chronic kidney disease in adult patients on dialysis.
Product Name : Vafseo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
December 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vafseo (vadadustat) is a once-daily oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor. It is approved for anemia due to chronic kidney disease in adult patients on dialysis.
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Vafseo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 07, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Akebia Provides Update on Vafseo® (vadadustat) Commercial Launch
Details : Vafseo (vadadustat) is a once-daily oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor. It is approved for anemia due to chronic kidney disease in adult patients on dialysis.
Product Name : Vafseo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
November 07, 2024

Details:
Undisclosed
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Akebia Therapeutics
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 25, 2024

Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Akebia Therapeutics
Deal Size : Inapplicable
Deal Type : Inapplicable
Vafseo Outcomes In-Center Experience
Details : Undisclosed
Product Name : Undisclosed
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The agreement aims to enable access to Vafseo (vadadustat), a once-daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor, for patients on dialysis.
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Vafseo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: US Renal Care
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement July 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : US Renal Care
Deal Size : Undisclosed
Deal Type : Agreement
Akebia And U.S. Renal Care Sign Contract For Vafseo® in Dialysis Patients
Details : The agreement aims to enable access to Vafseo (vadadustat), a once-daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor, for patients on dialysis.
Product Name : Vafseo
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
July 10, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vafseo (vadadustat) is a once-daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor. It is approved for the treatment of anemia due to chronic kidney disease in adult patients on dialysis.
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Vafseo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: US Renal Care
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 09, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : US Renal Care
Deal Size : Inapplicable
Deal Type : Inapplicable
Akebia Initiates VOICE Trial Of Vafseo® in Dialysis Patients
Details : Vafseo (vadadustat) is a once-daily oral hypoxia-inducible factor prolyl hydroxylase inhibitor. It is approved for the treatment of anemia due to chronic kidney disease in adult patients on dialysis.
Product Name : Vafseo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 09, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vafseo (vadadustat) is a once-daily oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor. It is approved for anemia due to chronic kidney disease in adult patients on dialysis.
Lead Product(s): Vadadustat,Inapplicable
Therapeutic Area: Nephrology Brand Name: Vafseo
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: CSL Vifor
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vadadustat,Inapplicable
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : CSL Vifor
Deal Size : Inapplicable
Deal Type : Inapplicable
Akebia Gets FDA Approval of Vafseo® for Anemia in Chronic Kidney Disease Patients
Details : Vafseo (vadadustat) is a once-daily oral hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor. It is approved for anemia due to chronic kidney disease in adult patients on dialysis.
Product Name : Vafseo
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
March 27, 2024

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : VAFSEO
Dosage Form : TABLET;ORAL
Dosage Strength : 150MG
Approval Date : 2024-03-27
Application Number : 215192
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : VAFSEO
Dosage Form : TABLET;ORAL
Dosage Strength : 300MG
Approval Date : 2024-03-27
Application Number : 215192
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : VAFSEO
Dosage Form : TABLET;ORAL
Dosage Strength : 450MG
Approval Date : 2024-03-27
Application Number : 215192
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
74
PharmaCompass offers a list of Vadadustat API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vadadustat manufacturer or Vadadustat supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vadadustat manufacturer or Vadadustat supplier.
PharmaCompass also assists you with knowing the Vadadustat API Price utilized in the formulation of products. Vadadustat API Price is not always fixed or binding as the Vadadustat Price is obtained through a variety of data sources. The Vadadustat Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A AKB-6548 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of AKB-6548, including repackagers and relabelers. The FDA regulates AKB-6548 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. AKB-6548 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of AKB-6548 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A AKB-6548 supplier is an individual or a company that provides AKB-6548 active pharmaceutical ingredient (API) or AKB-6548 finished formulations upon request. The AKB-6548 suppliers may include AKB-6548 API manufacturers, exporters, distributors and traders.
click here to find a list of AKB-6548 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a AKB-6548 Drug Master File in Korea (AKB-6548 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of AKB-6548. The MFDS reviews the AKB-6548 KDMF as part of the drug registration process and uses the information provided in the AKB-6548 KDMF to evaluate the safety and efficacy of the drug.
After submitting a AKB-6548 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their AKB-6548 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of AKB-6548 suppliers with KDMF on PharmaCompass.
AKB-6548 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of AKB-6548 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right AKB-6548 GMP manufacturer or AKB-6548 GMP API supplier for your needs.
A AKB-6548 CoA (Certificate of Analysis) is a formal document that attests to AKB-6548's compliance with AKB-6548 specifications and serves as a tool for batch-level quality control.
AKB-6548 CoA mostly includes findings from lab analyses of a specific batch. For each AKB-6548 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
AKB-6548 may be tested according to a variety of international standards, such as European Pharmacopoeia (AKB-6548 EP), AKB-6548 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (AKB-6548 USP).
