DMF filings rise 4.5% in Q3 2025; China holds lead, India records 20% growth in submissions
The
third quarter (Q3) of 2025 witnessed a steady rise in Drug Master File (DMF) submissions to the
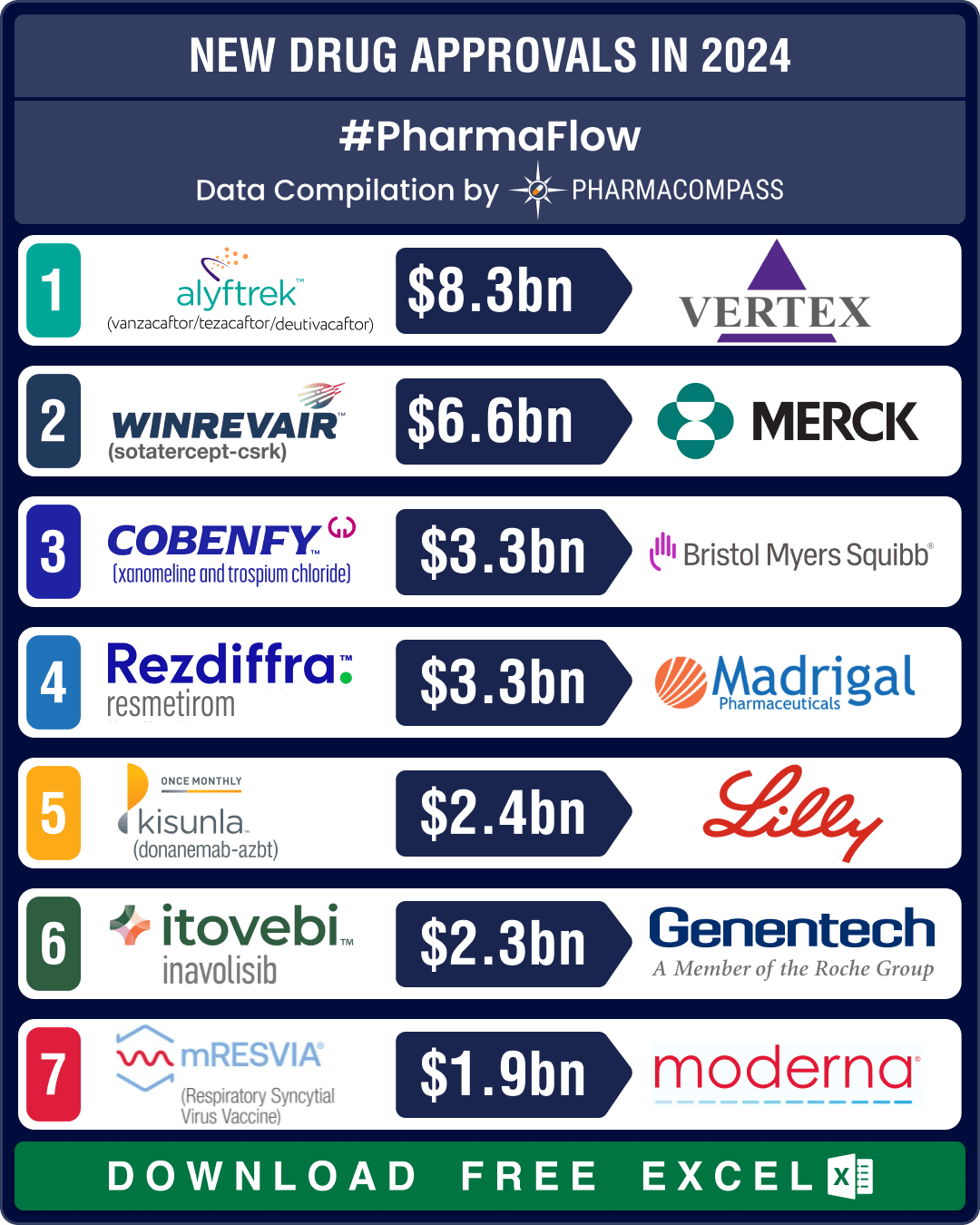
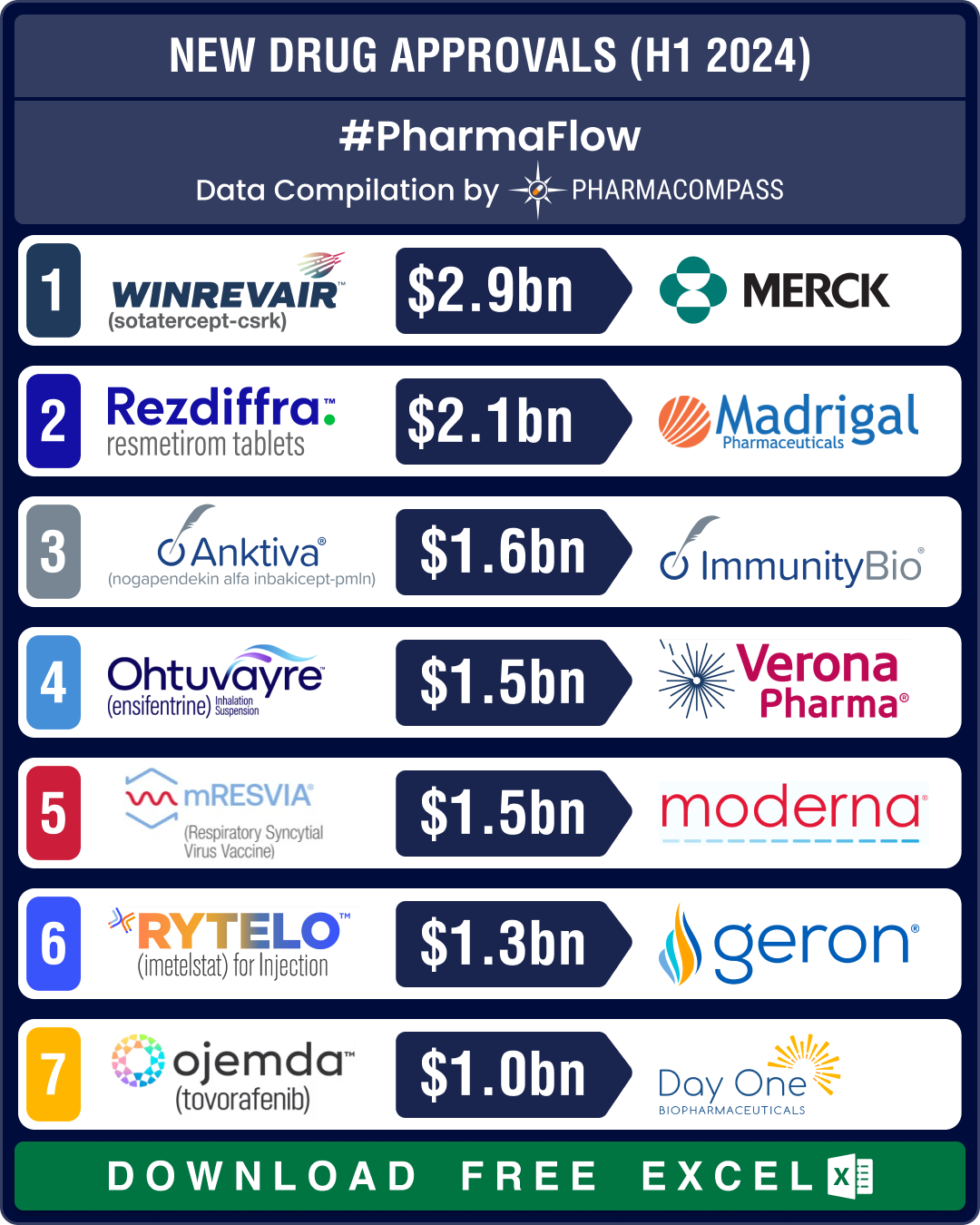
FDA okays 50 new drugs in 2024; BMS’ Cobenfy, Lilly’s Kisunla lead pack of breakthrough therapies
In 2024, the biopharma industry continued to advance on its robust trajectory of innovation. Though


 Market Place
Market Place Sourcing Support
Sourcing Support