
Synopsis
Synopsis
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
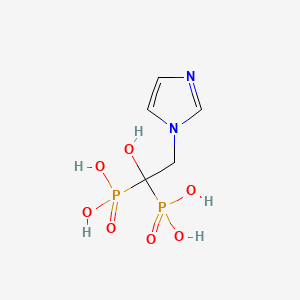

1. 2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosphonic Acid
2. Cgp 42'446
3. Cgp 42446
4. Cgp 42446a
5. Cgp-42'446
6. Cgp-42446
7. Cgp42'446
8. Cgp42446
9. Zoledronate
10. Zoledronic Acid Anhydrous
11. Zometa
1. Zoledronate
2. 118072-93-8
3. Zometa
4. Reclast
5. Aclasta
6. (1-hydroxy-2-(1h-imidazol-1-yl)ethane-1,1-diyl)diphosphonic Acid
7. Cgp 42446
8. (1-hydroxy-2-imidazol-1-ylethylidene)diphosphonic Acid
9. Zoledronic Acid Anhydrous
10. Anhydrous Zoledronic Acid
11. (1-hydroxy-2-imidazol-1-yl-1-phosphonoethyl)phosphonic Acid
12. Phosphonic Acid, [1-hydroxy-2-(1h-imidazol-1-yl)ethylidene]bis-
13. Zol
14. [1-hydroxy-2-(1h-imidazol-1-yl)ethane-1,1-diyl]bis(phosphonic Acid)
15. Orazol
16. Zol 446
17. Zoledronic Acid (inn)
18. Cgp-42446
19. Reclast (tn)
20. Zometa (tn)
21. Chembl924
22. Zoledronic Acid Teva
23. Zoledronic Acid, Anhydrous
24. Nsc-721517
25. Zoledronic Acid Medac
26. Chebi:46557
27. [1-hydroxy-2-(1h-imidazol-1-yl)-1-phosphonoethyl]phosphonic Acid
28. 70hz18ph24
29. Ncgc00159521-02
30. (1-hydroxy-2-(1h-imidazol-1-yl)ethylidene)bisphosphonic Acid
31. Cgp-42446a
32. Zoledronate Hydrate
33. Zoledronic Acid [usan:inn:ban]
34. Phosphonic Acid, (1-hydroxy-2-(1h-imidazol-1-yl)ethylidene)bis-
35. Zoladrona Acid Mylan
36. Zoledronic
37. Zoledronic Acid Accord
38. Zoledronic Acid [inn]
39. Zomera
40. 1-hydroxy-2-(1h-imidazol-1-yl)ethane-1,1-diyldiphosphonic Acid
41. Bisphosphonate 3
42. Zometa (novartis)
43. Aclasta And Reclast
44. C5h10n2o7p2
45. [1-hydroxy-2-(1h-imidazol-1-yl)ethylidene]bisphosphonic Acid
46. Zoledronic-acid
47. Unii-70hz18ph24
48. Bph 91
49. [1-hydroxy-2-(1h-imidazol-1-yl)-ethylidene]bisphosphonic Acid
50. Dsstox_cid_22668
51. Dsstox_rid_80065
52. Zoledronic Acid, Zoledronate
53. Bidd:pxr0134
54. Dsstox_gsid_42668
55. Schembl19054
56. Zoledronic Acid [mi]
57. Bidd:gt0292
58. Zoledronic Acid (zoledronate)
59. Gtpl3177
60. Jmc515594 Compound 55
61. Dtxsid0042668
62. Bdbm12578
63. Cgp42446a
64. Zoledronic Acid [who-dd]
65. Hms2089o09
66. Bcp22750
67. Cgp-4244
68. Zinc3803652
69. Tox21_111739
70. Mfcd00867791
71. Nsc721517
72. S1314
73. Stl452893
74. Akos005145739
75. Ab07564
76. Ac-1092
77. Cs-1829
78. Db00399
79. Hs-0091
80. Nsc 721517
81. Ncgc00159521-03
82. Ncgc00159521-04
83. Ncgc00159521-05
84. Ncgc00159521-09
85. Ncgc00159521-18
86. Hy-13777
87. Cas-118072-93-8
88. Ft-0601384
89. Z0031
90. D08689
91. H11422
92. S00092
93. Ab01273947-01
94. Ab01273947-02
95. Ab01273947-03
96. Ab01273947_04
97. 072z938
98. A803876
99. Q218507
100. Sr-05000001436
101. Q-201946
102. Sr-05000001436-1
103. 1-hydroxy-2-(1-imidazolyl)ethane-1,1-diphosphonic Acid
104. Z1691545083
105. (1-hydroxy-2-(1h-imidazol-1-yl)ethane-1,1-diyl)diphosphonicacid
| Molecular Weight | 272.09 g/mol |
|---|---|
| Molecular Formula | C5H10N2O7P2 |
| XLogP3 | -4.3 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 271.99632466 g/mol |
| Monoisotopic Mass | 271.99632466 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 327 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Reclast |
| Drug Label | Reclast contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formul... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion) |
| Strength | eq 5mg base/100ml; 5mg/100ml |
| Market Status | Prescription |
| Company | Novartis Pharms; Novartis |
| 2 of 6 | |
|---|---|
| Drug Name | Zoledronic acid |
| PubMed Health | Zoledronic Acid (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Zoledronic Acid Injection contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion); Injection; iv (infusion) |
| Strength | eq 5mg base/100ml; 5mg/100ml; 4mmg; eq 4mg base/100ml; eq 4mg base/5ml; 4mg/100ml; 4mg/5ml(0.8mg/ml); eq 4mg base/vial |
| Market Status | Tentative Approval; Prescription |
| Company | Pharmaceutics; Hospira; Gland Pharma; Teva Parenteral; Apotex; Hikma Farmaceutica; Usv North America; Acs Dobfar Info Sa; Pharmaforce; Cipla; Sun Pharma Global; Emcure Pharms; Pharms; Dr Reddys Labs; Agila Speclts; Actavis; Akorn |
| 3 of 6 | |
|---|---|
| Drug Name | Zometa |
| PubMed Health | Zoledronic Acid (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Zometa contains zoledronicacid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronicacid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formu... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | Iv (infusion); iv (infusion) |
| Strength | eq 4mg base/100ml; eq 4mg base/5ml; eq 4mg base/vial |
| Market Status | Prescription |
| Company | Novartis |
| 4 of 6 | |
|---|---|
| Drug Name | Reclast |
| Drug Label | Reclast contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formul... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion) |
| Strength | eq 5mg base/100ml; 5mg/100ml |
| Market Status | Prescription |
| Company | Novartis Pharms; Novartis |
| 5 of 6 | |
|---|---|
| Drug Name | Zoledronic acid |
| PubMed Health | Zoledronic Acid (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Zoledronic Acid Injection contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | injection; Iv (infusion); Injection; iv (infusion) |
| Strength | eq 5mg base/100ml; 5mg/100ml; 4mmg; eq 4mg base/100ml; eq 4mg base/5ml; 4mg/100ml; 4mg/5ml(0.8mg/ml); eq 4mg base/vial |
| Market Status | Tentative Approval; Prescription |
| Company | Pharmaceutics; Hospira; Gland Pharma; Teva Parenteral; Apotex; Hikma Farmaceutica; Usv North America; Acs Dobfar Info Sa; Pharmaforce; Cipla; Sun Pharma Global; Emcure Pharms; Pharms; Dr Reddys Labs; Agila Speclts; Actavis; Akorn |
| 6 of 6 | |
|---|---|
| Drug Name | Zometa |
| PubMed Health | Zoledronic Acid (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Zometa contains zoledronicacid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronicacid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formu... |
| Active Ingredient | Zoledronic acid |
| Dosage Form | Injectable |
| Route | Iv (infusion); iv (infusion) |
| Strength | eq 4mg base/100ml; eq 4mg base/5ml; eq 4mg base/vial |
| Market Status | Prescription |
| Company | Novartis |
Zoledronic acid is indicated to treat hypercalcemia of malignancy, multiple myeloma, bone metastases from solid tumors, osteoporosis in men and postmenopausal women, glucocorticoid induced osteoporosis, and Paget's disease of bone in men and women. Zoledronic acid is also indicated for the prevention of osteoporosis in post menopausal women and glucocorticoid induced osteoporosis.
Prevention of skeletal-related events and treatment of tumour-induced hypercalcaemia.
Prevention of skeletal-related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.
Treatment of adult patients with tumour-induced hypercalcaemia.
* 4 mg / 5 ml and 4 mg / 100 ml: :
- Prevention of skeletal-related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.
- Treatment of adult patients with tumour-induced hypercalcaemia (TIH).
* 5 mg / 100 ml: :
Treatment of osteoporosis:
- in post-menopausal women;
- in men;
at increased risk of fracture, including those with a recent low-trauma hip fracture.
Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy:
- in post-menopausal women;
- in men;
at increased risk of fracture.
Treatment of Paget's disease of the bone in adults.
Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.
Treatment of adult patients with tumour-induced hypercalcaemia (TIH).
- Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone;
- treatment of adult patients with tumour-induced hypercalcaemia (TIH).
Treatment of osteoporosis:
- in post-menopausal women;
- in men;
at increased risk of fracture, including those with a recent low-trauma hip fracture.
Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy in post-menopausal women and in men at increased risk of fracture.
Treatment of Paget's disease of the bone.
Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone.
Treatment of adult patients with tumour-induced hypercalcaemia (TIH).
Treatment of osteoporosis:
- in post-menopausal women;
- in men;
at increased risk of fracture including those with a recent low-trauma hip fracture.
Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy:
- in post-menopausal women;
- in men;
at increased risk of fracture.
Treatment of Pagets disease of the bone in adults.
- Prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in patients with advanced malignancies involving bone;
- treatment of tumour-induced hypercalcaemia (TIH);
- prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in patients with advanced malignancies involving bone;
- treatment of tumour-induced hypercalcaemia (TIH);
- prevention of skeletal related events (pathological fractures, spinal compression, radiation or surgery to bone, or tumour-induced hypercalcaemia) in adult patients with advanced malignancies involving bone;
- treatment of adult patients with tumour-induced hypercalcaemia (TIH).
Treatment of osteoporosis
- in post-menopausal women
- in adult men
at increased risk of fracture, including those with recent low-trauma hip fracture.
Treatment of osteoporosis associated with long-term systemic glucocorticoid therapy
- in post-menopausal women
- in adult men
at increased risk of fracture.
Treatment of Paget's disease of the bone in adults.
Osteogenesis imperfecta, Prevention of fracture and bone loss in postmenopausal women with early-stage breast cancer treated with aromatase inhibitors, Prevention of skeletal related events in patients with advanced malignancies involving bone, Tumour-induced hypercalcaemia
Treatment of osteoporosis, Treatment of Pagets disease of the bone
Zoledronic acid is a third generation, nitrogen containing bisphosphonate that inhibits osteoclast function and prevents bone resorption. The therapeutic window is wide as patients are unlikely to suffer severe effects from overdoses and the duration of action is long. Patients should be counselled regarding the risk of electrolyte deficiencies, renal impairment, osteonecrosis of the jaw, atypical femoral fractures, bronchoconstriction, hepatic impairment, hypocalcemia, and embryo-fetal toxicity.
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
M05BA08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M05 - Drugs for treatment of bone diseases
M05B - Drugs affecting bone structure and mineralization
M05BA - Bisphosphonates
M05BA08 - Zoledronic acid
Absorption
A 4mg intravenous dose reaches a Cmax of 37078.5ng/mL, with a Tmax of 0.3170.014h, and an AUC of 788181ng\*h/mL. A 5mg intravenous dose reaches a Cmax of 47176.1ng/mL, with a Tmax of 0.3680.005h, and an AUC of 917226ng\*h/mL.
Route of Elimination
Zoledronic acid is 39 16% eliminated in the urine as the unmetabolized parent drug.
Clearance
Zoledronic acid has a renal clearance of 3.7 2.0 L/h.
Zoledronic acid is not metabolized _in vivio_.
Zoledronic acid has a terminal elimination half life of 146 hours.
Bisphosphonates are taken into the bone where they bind to hydroxyapatite. Bone resorption by osteoclasts causes local acidification, releasing the bisphosphonate, which is taken into the osteoclast by fluid-phase endocytosis. Endocytic vesicles become acidified, releasing bisphosphonates into the cytosol of osteoclasts where they act. Osteoclasts mediate resorption of bone. When osteoclasts bind to bone they form podosomes, ring structures of F-actin. Etidronic acid also inhibits V-ATPases in the osteoclast, though the exact subunits are unknown, preventing F-actin from forming podosomes. Disruption of the podosomes causes osteoclasts to detach from bones, preventing bone resorption. Nitrogen containing bisphosphonates such as zoledronate are known to induce apoptosis of hematopoietic tumor cells by inhibiting the components of the mevalonate pathway farnesyl diphosphate synthase, farnesyl diphosphate, and geranylgeranyl diphosphate. These components are essential for post-translational prenylation of GTP-binding proteins like Rap1. The lack of prenylation of these proteins interferes with their function, and in the case of Rap1, leads to apoptosis. zoledronate also activated caspases which further contribute to apoptosis.
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2016-06-03
Pay. Date : 2015-08-27
DMF Number : 24306
Submission : 2010-10-13
Status : Active
Type : II

Certificate Number : CEP 2018-191 - Rev 03
Issue Date : 2024-07-23
Type : Chemical
Substance Number : 2743
Status : Valid

Registration Number : 225MF10034
Registrant's Address : 19 Pellinska Str. 83-200 Starogard Gdanski POLAND
Initial Date of Registration : 2013-02-20
Latest Date of Registration :

| Available Reg Filing : ASMF, CA |
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21344
Submission : 2008-02-18
Status : Active
Type : II

Date of Issue : 28-05-2025
Valid Till : 25-06-2028
Written Confirmation Number : WC-0035
Address of the Firm :

NDC Package Code : 55111-813
Start Marketing Date : 2008-02-18
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
Polfa Tarchomin is a leading Polish pharmaceutical company with 200 year tradition in manufacture and sale of pharmaceutical products.
 Synnat Pharma is one of the leading active pharmaceutical ingredients and intermediates manufacturers.
Synnat Pharma is one of the leading active pharmaceutical ingredients and intermediates manufacturers.

Date of Issue : 2024-05-22
Valid Till : 2027-07-02
Written Confirmation Number : WC-0301
Address of the Firm :
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : Reviewed
Rev. Date : 2012-11-23
Pay. Date : 2012-11-13
DMF Number : 19701
Submission : 2006-08-22
Status : Active
Type : II

| Available Reg Filing : ASMF |

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40024
Submission : 2024-05-29
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Company : Novartis/Sandoz
Zoledronic Acid/Mannitol-Water
Drug Cost (USD) : 85,278
Year : 2023
Prescribers : 82
Prescriptions : 82

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Sagent Pharmace
Zoledronic Ac/Mannitol/0.9nacl
Drug Cost (USD) : 73,051
Year : 2023
Prescribers : 177
Prescriptions : 466

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Sagent-Novaplus
Zoledronic Ac/Mannitol/0.9nacl
Drug Cost (USD) : 728
Year : 2023
Prescribers :
Prescriptions : 11

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Accord Healthca
Zoledronic Acid
Drug Cost (USD) : 526,220
Year : 2023
Prescribers : 527
Prescriptions : 1740

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : App/Fresenius K
Zoledronic Acid
Drug Cost (USD) : 1,605
Year : 2023
Prescribers :
Prescriptions : 12

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Bluepoint Labor
Zoledronic Acid
Drug Cost (USD) : 91,591
Year : 2023
Prescribers : 174
Prescriptions : 447

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Bpi Labs, LLC
Zoledronic Acid
Drug Cost (USD) : 4,705
Year : 2023
Prescribers : 94
Prescriptions : 148

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Dr. Reddy'S-Nov
Zoledronic Acid
Drug Cost (USD) : 354
Year : 2023
Prescribers :
Prescriptions : 11

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Dr.Reddy'S Lab
Zoledronic Acid
Drug Cost (USD) : 70,728
Year : 2023
Prescribers : 465
Prescriptions : 1173

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company : Hospira/Pfizer
Zoledronic Acid
Drug Cost (USD) : 3,888
Year : 2023
Prescribers : 48
Prescriptions : 94

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Concentrate to the infusion flui...
Dosage Strength : 4 mg/5 ml
Price Per Pack (Euro) : 175.97
Published in :
Country : Norway
RX/OTC/DISCN :

The Fresenius Kabi Norge AS - Halde...
Dosage Form : Concentrate to the infusion flui...
Dosage Strength : 4 mg/5 ml
Price Per Pack (Euro) : 175.97
Published in :
Country : Norway
RX/OTC/DISCN :

Dosage Form : Inf Conc
Dosage Strength : 4mg/5ml
Price Per Pack (Euro) : 105.85
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

Fresenius Kabi (Switzerland) AG
Dosage Form : Inf Conc
Dosage Strength : 4mg/5ml
Price Per Pack (Euro) : 98.47
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

Dosage Form : Inf Conc
Dosage Strength : 4mg/5ml
Price Per Pack (Euro) : 108.31
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

Dosage Form : Inf Conc
Dosage Strength : 4mg/5ml
Price Per Pack (Euro) : 165.83
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

Dosage Form : Inf Conc
Dosage Strength : 4mg/5ml
Price Per Pack (Euro) : 108.31
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

Dosage Form : Inf Conc
Dosage Strength : 4mg/5ml
Price Per Pack (Euro) : 108.31
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

Dosage Form : Inf L?s
Dosage Strength : 5mg/100ml
Price Per Pack (Euro) : 214.03
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

Dosage Form : Inf L?s
Dosage Strength : 5mg/100ml
Price Per Pack (Euro) : 214.03
Published in :
Country : Switzerland
RX/OTC/DISCN : Class B

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Main Therapeutic Indication : Oncology
Currency : USD
2014 Revenue in Millions : -56.00%
2013 Revenue in Millions :
Growth (%) :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
13 Feb 2025
Reply
02 Nov 2023
Reply
24 Aug 2023
Reply
09 Aug 2023
Reply
02 May 2023
Reply
10 Aug 2022
Reply
25 Jan 2021
Reply
10 Feb 2020
Reply
17 May 2019
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
87
PharmaCompass offers a list of Zoledronic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Zoledronic Acid manufacturer or Zoledronic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Zoledronic Acid manufacturer or Zoledronic Acid supplier.
PharmaCompass also assists you with knowing the Zoledronic Acid API Price utilized in the formulation of products. Zoledronic Acid API Price is not always fixed or binding as the Zoledronic Acid Price is obtained through a variety of data sources. The Zoledronic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Zoledronic Acid Hydrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Zoledronic Acid Hydrate, including repackagers and relabelers. The FDA regulates Zoledronic Acid Hydrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Zoledronic Acid Hydrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Zoledronic Acid Hydrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Zoledronic Acid Hydrate supplier is an individual or a company that provides Zoledronic Acid Hydrate active pharmaceutical ingredient (API) or Zoledronic Acid Hydrate finished formulations upon request. The Zoledronic Acid Hydrate suppliers may include Zoledronic Acid Hydrate API manufacturers, exporters, distributors and traders.
click here to find a list of Zoledronic Acid Hydrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Zoledronic Acid Hydrate DMF (Drug Master File) is a document detailing the whole manufacturing process of Zoledronic Acid Hydrate active pharmaceutical ingredient (API) in detail. Different forms of Zoledronic Acid Hydrate DMFs exist exist since differing nations have different regulations, such as Zoledronic Acid Hydrate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Zoledronic Acid Hydrate DMF submitted to regulatory agencies in the US is known as a USDMF. Zoledronic Acid Hydrate USDMF includes data on Zoledronic Acid Hydrate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Zoledronic Acid Hydrate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Zoledronic Acid Hydrate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Zoledronic Acid Hydrate Drug Master File in Japan (Zoledronic Acid Hydrate JDMF) empowers Zoledronic Acid Hydrate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Zoledronic Acid Hydrate JDMF during the approval evaluation for pharmaceutical products. At the time of Zoledronic Acid Hydrate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Zoledronic Acid Hydrate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Zoledronic Acid Hydrate Drug Master File in Korea (Zoledronic Acid Hydrate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Zoledronic Acid Hydrate. The MFDS reviews the Zoledronic Acid Hydrate KDMF as part of the drug registration process and uses the information provided in the Zoledronic Acid Hydrate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Zoledronic Acid Hydrate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Zoledronic Acid Hydrate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Zoledronic Acid Hydrate suppliers with KDMF on PharmaCompass.
A Zoledronic Acid Hydrate CEP of the European Pharmacopoeia monograph is often referred to as a Zoledronic Acid Hydrate Certificate of Suitability (COS). The purpose of a Zoledronic Acid Hydrate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Zoledronic Acid Hydrate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Zoledronic Acid Hydrate to their clients by showing that a Zoledronic Acid Hydrate CEP has been issued for it. The manufacturer submits a Zoledronic Acid Hydrate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Zoledronic Acid Hydrate CEP holder for the record. Additionally, the data presented in the Zoledronic Acid Hydrate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Zoledronic Acid Hydrate DMF.
A Zoledronic Acid Hydrate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Zoledronic Acid Hydrate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Zoledronic Acid Hydrate suppliers with CEP (COS) on PharmaCompass.
A Zoledronic Acid Hydrate written confirmation (Zoledronic Acid Hydrate WC) is an official document issued by a regulatory agency to a Zoledronic Acid Hydrate manufacturer, verifying that the manufacturing facility of a Zoledronic Acid Hydrate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Zoledronic Acid Hydrate APIs or Zoledronic Acid Hydrate finished pharmaceutical products to another nation, regulatory agencies frequently require a Zoledronic Acid Hydrate WC (written confirmation) as part of the regulatory process.
click here to find a list of Zoledronic Acid Hydrate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Zoledronic Acid Hydrate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Zoledronic Acid Hydrate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Zoledronic Acid Hydrate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Zoledronic Acid Hydrate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Zoledronic Acid Hydrate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Zoledronic Acid Hydrate suppliers with NDC on PharmaCompass.
Zoledronic Acid Hydrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Zoledronic Acid Hydrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Zoledronic Acid Hydrate GMP manufacturer or Zoledronic Acid Hydrate GMP API supplier for your needs.
A Zoledronic Acid Hydrate CoA (Certificate of Analysis) is a formal document that attests to Zoledronic Acid Hydrate's compliance with Zoledronic Acid Hydrate specifications and serves as a tool for batch-level quality control.
Zoledronic Acid Hydrate CoA mostly includes findings from lab analyses of a specific batch. For each Zoledronic Acid Hydrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Zoledronic Acid Hydrate may be tested according to a variety of international standards, such as European Pharmacopoeia (Zoledronic Acid Hydrate EP), Zoledronic Acid Hydrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Zoledronic Acid Hydrate USP).
