
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
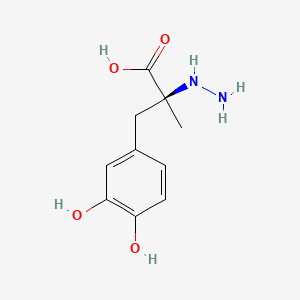

1. Carbidopa, (r)-isomer
2. Carbidopa, (s)-isomer
3. Lodosin
4. Lodosyn
5. Methyldopahydrazine
6. Mk 485
7. Mk 486
8. Mk-485
9. Mk-486
10. Mk485
11. Mk486
1. 28860-95-9
2. Lodosyn
3. Carbidopa Anhydrous
4. (s)-(-)-carbidopa
5. (s)-carbidopa
6. S-(-)-carbidopa
7. Alpha-methyldopahydrazine
8. L-alpha-methyldopahydrazine
9. N-aminomethyldopa
10. Carbidopum
11. Carbidopum [inn-latin]
12. Carbidopa (anhydrous)
13. S(-)-carbidopa
14. Mk 486
15. (2s)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic Acid
16. Carbidopa [inn]
17. L-3-(3,4-dihydroxyphenyl)-2-methyl-2-hydrazinopropionic Acid
18. (2s)-3-(3,4-dihydroxyphenyl)-2-hydrazino-2-methylpropanoic Acid
19. (-)-l-alpha-hydrazino-3,4-dihydroxy-alpha-methylhydrocinnamic Acid
20. (s)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic Acid
21. Kr87b45rgh
22. (alphas)-alpha-hydrazino-3,4-dihydroxy-alpha-methylbenzenepropanoic Acid
23. Chebi:39585
24. 28860-95-9 (anhydrous)
25. Ncgc00024596-05
26. Hadrazino-alpha-methyldopa
27. C-dopa
28. 3,3,3-trideuterio-2-[dideuterio-(3,4-dihydroxyphenyl)methyl]-2-hydrazinylpropanoic Acid
29. Smr000058235
30. Ccris 5093
31. Sr-01000597655
32. Einecs 249-271-9
33. Unii-kr87b45rgh
34. Benzenepropanoic Acid, .alpha.-hydrazino-3,4-dihydroxy-.alpha.-methyl-, (s)-
35. Alpha-hydrazino-alpha-methyl-beta-(3,4-dihydroxyphenyl)propionic Acid
36. L-alpha-methyl-alpha-hydrazino-beta-(3,4-dihydroxyphenylpropionic Acid
37. L-alpha-methyl-beta-(3,4-dihydroxyphenyl)-alpha-hydrazinopropionic Acid
38. Mfcd00069231
39. Nd0611
40. Tocris-0455
41. C-126
42. Dsstox_cid_2735
43. Carbidopa [who-dd]
44. Dsstox_rid_76707
45. Dsstox_gsid_22735
46. Lopac0_000382
47. Schembl35084
48. Mls000069628
49. Mls002207014
50. S-(-)-carbidopa Monohydrate
51. Gtpl5159
52. Carbidopa, L- Anhydrous
53. Carbidopa Anhydrous [mi]
54. Chembl1201236
55. Dtxsid4022735
56. Hms2089b12
57. Hms3266a20
58. Hms3411m13
59. Hms3655g20
60. Hms3675m13
61. Hms3713l10
62. Hms3884m14
63. Hy-b0311
64. Tox21_110910
65. (2s)-3-(3,4-dihydroxyphenyl)-2-hydrazino-2-methyl-propanoic Acid
66. Bdbm50418773
67. Nsc751137
68. S1891
69. Zinc19168887
70. Akos015969657
71. Benzenepropanoic Acid, Alpha-hydrazino-3,4-dihydroxy-alpha-methyl-, (s)-
72. Hydrocinnamic Acid, Alpha-hydrazino-3,4-dihydroxy-alpha-methyl-, L-
73. Ac-1676
74. Ccg-204476
75. Db00190
76. Sdccgmls-0072919.p025
77. Mls-0072919
78. Smp1_000057
79. Ncgc00024596-01
80. Ncgc00024596-03
81. Ncgc00024596-06
82. Ncgc00024596-07
83. Ncgc00024596-08
84. As-16862
85. Bc164279
86. Mls-0072919.p013
87. Cas-28860-95-9
88. Eu-0100382
89. Sw199080-2
90. 60c959
91. Ab00441332-05
92. Ab00441332-06
93. Ab00441332_07
94. Ab00441332_08
95. Sr-01000597655-1
96. Sr-01000597655-3
97. Sr-01000597655-5
98. Sr-01000597655-9
99. Brd-k78712176-001-07-5
100. Benzenepropanoic Acid, A-hydrazino-3,4-dihydroxy-a-methyl-
101. (-)-l-alpha-hydrazino-3,4-dihydroxy-alpha-methylhydrocinamic Acid
102. (s)-?-hydrazino-?-methyl-?-(3,4-dihydroxyphenyl)propionic Acid
103. (s)-3-(3,4-dihydroxyphenyl)-2-hydrazino-2-methylpropionic Acid
104. (s)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoicacid
105. (2s)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methyl-propanoic Acid
106. (2s)-3-(3,4-dihydroxyphenyl)-2-hydrazino-2-methylpropionic Acid Monohydrate
107. Benzenepropanoic Acid, .alpha.-hydrazinyl-3,4-dihydroxy-.alpha.-methyl-, (.alpha.s)-
108. Kinson; 3-(3,4-dihydroxy-phenyl)-2-hydrazino-2-methyl-propionic Acid
109. 1426847-87-1
| Molecular Weight | 226.23 g/mol |
|---|---|
| Molecular Formula | C10H14N2O4 |
| XLogP3 | -2.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 226.09535693 g/mol |
| Monoisotopic Mass | 226.09535693 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 261 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Carbidopa |
| PubMed Health | Carbidopa (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | Carbidopa and levodopa extended release tablets are extended release combination of carbidopa and levodopa for the treatment of Parkinsons disease and syndrome.Carbidopa, an inhibitor of aromatic amino acid decarboxylation, is a white, crystalline... |
| Active Ingredient | Carbidopa |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Amerigen Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Lodosyn |
| PubMed Health | Carbidopa (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | Carbidopa, an inhibitor of aromatic amino acid decarboxylation, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 244.3. It is designated chemically as ()-L--hydrazino--methyl--(3,4-dihydroxybenzene) pro... |
| Active Ingredient | Carbidopa |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Aton |
| 3 of 4 | |
|---|---|
| Drug Name | Carbidopa |
| PubMed Health | Carbidopa (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | Carbidopa and levodopa extended release tablets are extended release combination of carbidopa and levodopa for the treatment of Parkinsons disease and syndrome.Carbidopa, an inhibitor of aromatic amino acid decarboxylation, is a white, crystalline... |
| Active Ingredient | Carbidopa |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Amerigen Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Lodosyn |
| PubMed Health | Carbidopa (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | Carbidopa, an inhibitor of aromatic amino acid decarboxylation, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 244.3. It is designated chemically as ()-L--hydrazino--methyl--(3,4-dihydroxybenzene) pro... |
| Active Ingredient | Carbidopa |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg |
| Market Status | Prescription |
| Company | Aton |
Carbidopa is indicated with [levodopa] for the treatment of symptoms of idiopathic Parkinson disease, postencephalitic parkinsonism and symptomatic parkinsonism followed by carbon monoxide or manganese intoxication. The combination therapy is administered for the reduction of [levodopa]-driven nausea and vomiting. The product of carbidopa should be used in patients where the combination therapy of carbidopa/[levodopa] provide less than the adequate daily dosage. As well carbidopa can be used in patients where the dosages of carbidopa and [levodopa] require individual titration.
FDA Label
When mixed with [levodopa], carbidopa inhibits the peripheral conversion of [levodopa] to dopamine and the decarboxylation of [oxitriptan] to serotonin by aromatic L-amino acid decarboxylase. This results in an increased amount of [levodopa] and [oxitriptan] available for transport to the central nervous system. Carbidopa also inhibits the metabolism of [levodopa] in the GI tract, thus, increasing the bioavailability of [levodopa]. The presence of additional units of circulating [levodopa] can increase the effectiveness of the still functional dopaminergic neurons and it has been shown to alleviate symptoms for a time. The action of carbidopa is very important as [levodopa] is able to cross the blood-brain barrier while dopamine cannot. Hence the administration of carbidopa is essential to prevent the transformation of external [levodopa] to dopamine before reaching the main action site in the brain. The coadministration of carbidopa with [levodopa] has been shown to increase the half-life of [levodopa] more than 1.5 times while increasing the plasma level and decreasing clearance. The combination therapy has also shown an increase of the recovery of [levodopa] in urine instead of dopamine which proves a reduced metabolism. This effect has been highly observed by a significant reduction in [levodopa] requirements and a significant reduction in the presence of side effects such as nausea. It has been observed that the effect of carbidopa is not dose-dependent.
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Aromatic Amino Acid Decarboxylase Inhibitors
Compounds and drugs that block or inhibit the enzymatic action of AROMATIC AMINO ACID DECARBOXYLASES. Pharmaceutical agents in this category are used in conjunction with LEVODOPA in order to slow its metabolism. (See all compounds classified as Aromatic Amino Acid Decarboxylase Inhibitors.)
Dopamine Agents
Any drugs that are used for their effects on dopamine receptors, on the life cycle of dopamine, or on the survival of dopaminergic neurons. (See all compounds classified as Dopamine Agents.)
Absorption
When [levodopa]/carbidopa is administered orally, 40-70% of the administered dose is absorbed. Once absorbed, carbidopa shows bioavailability of 58%. A maximum concentration of 0.085 mcg/ml was achieved after 143 min with an AUC of 19.28 mcg.min/ml.
Route of Elimination
In animal studies, 66% of the administered dose of carbidopa was eliminated via the urine while 11% was found in feces. These studies were performed in humans and it was observed a urine excretion covering 50% of the administered dose.
Volume of Distribution
The volume of distribution reported for the combination therapy of carbidopa/[levodopa] is of 3.6 L/kg. However, carbidopa is widely distributed in the tissues, except in the brain. After one hour, carbidopa is found mainly in the kidney, lungs, small intestine and liver.
Clearance
The reported clearance rate for the combination therapy of [levodopa]/carbidopa is 51.7 L/h.
The loss of the hydrazine functional group (probably as molecular nitrogen) represents the major metabolic pathway for carbidopa. There are several metabolites of carbidopa metabolism including 3-(3,4-dihydroxyphenyl)-2-methylpropionic acid, 3-(4-hydroxy-3-methoxyphenyl)-2-methylpropionic acid, 3-(3-hydroxyphenyl)-2-methylpropionic acid, 3-(4-hydroxy-3-methoxyphenyl)-2-methyllactic acid, 3-(3-hydroxyphenyl)-2-methyllactic acid, and 3,4-dihydroxyphenylacetone (1,2).
The reported half-life of carbidopa is of approximately 107 minutes.
Carbidopa is an inhibitor of the DDC which in order, inhibits the peripheral metabolism of levodopa. DDC is very important in the biosynthesis of L-tryptophan to serotonin and the modification of L-DOPA to dopamine. DDC can be found in the body periphery and in the blood-brain barrier. The action of carbidopa is focused on peripheral DDC as this drug cannot cross the blood-brain barrier. Hence, it will prevent the metabolism of [levodopa] in the periphery but it will not have any activity on the generation of dopamine in the brain.
 Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-07-11
Pay. Date : 2013-06-17
DMF Number : 9231
Submission : 1991-07-11
Status : Active
Type : II

Certificate Number : R1-CEP 2000-012 - Rev 08
Issue Date : 2019-12-16
Type : Chemical
Substance Number : 755
Status : Valid

Registration Number : 222MF10146
Registrant's Address : Route du simplon 22, CH-1895 Vionnaz-Switzerland
Initial Date of Registration : 2010-04-22
Latest Date of Registration :

NDC Package Code : 51504-0001
Start Marketing Date : 1991-07-11
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Kukjeon Pharmaceutical Co., Ltd.
Registration Date : 2021-03-03
Registration Number : 20210303-211-J-655
Manufacturer Name : Bachem SA
Manufacturer Address : Succursale of Vionnaz, Route du Simplon 22, 1895 Vionnaz, Switzerland


GDUFA
DMF Review : Reviewed
Rev. Date : 2013-11-20
Pay. Date : 2013-05-10
DMF Number : 26416
Submission : 2012-09-14
Status : Active
Type : II

Certificate Number : CEP 2011-275 - Rev 02
Issue Date : 2024-09-25
Type : Chemical
Substance Number : 755
Status : Valid

NDC Package Code : 12780-4420
Start Marketing Date : 2011-06-28
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Alvogen Korea Co., Ltd.
Registration Date : 2022-01-10
Registration Number : 20220110-211-J-1205
Manufacturer Name : Fermion Oy Hanko Plant@Zhejiang Chiral Medicine Chemicals Co., Ltd@Zhejiang Yongtai Chiral Medicine Technology Co., Ltd.
Manufacturer Address : Orioninkatu 2, Hanko Pohjoinen, 10960, Finland@Nanyang Economy Development Zone, Xiaoshan, Hangzhou, Zhejiang Province, 311227, China@No.7 , Donghai 4th Road, Taizhou Bay Economic and Technological Development Zone, Linhai, Taizhou, Zhejiang Province, China

| Available Reg Filing : BR |
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.

Registration Number : 220MF10226
Registrant's Address : H-1106 BUDAPEST KERESZTURI UT 30-38 HUNGARY
Initial Date of Registration : 2008-11-10
Latest Date of Registration :

 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : Reviewed
Rev. Date : 2012-11-29
Pay. Date : 2012-11-13
DMF Number : 8296
Submission : 1989-11-14
Status : Active
Type : II

Certificate Number : CEP 2002-052 - Rev 09
Issue Date : 2025-02-25
Type : Chemical
Substance Number : 755
Status : Valid

Date of Issue : 2025-04-24
Valid Till : 2028-05-25
Written Confirmation Number : WC-0002
Address of the Firm :

NDC Package Code : 15894-0040
Start Marketing Date : 2023-02-23
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : CA, ASMF |


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35521
Submission : 2020-12-31
Status : Active
Type : II

Certificate Number : R0-CEP 2020-143 - Rev 00
Issue Date : 2021-06-11
Type : Chemical
Substance Number : 755
Status : Valid

Date of Issue : 2024-02-12
Valid Till : 2027-02-11
Written Confirmation Number : WC-0407
Address of the Firm :

NDC Package Code : 70600-018
Start Marketing Date : 2020-12-15
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : ASMF, CN, BR |

 Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Certificate Number : R0-CEP 2020-364 - Rev 00
Issue Date : 2021-06-22
Type : Chemical
Substance Number : 755
Status : Withdrawn by Holder


Certificate Number : CEP 2025-265 - Rev 00
Issue Date : 2025-07-29
Type : Chemical
Substance Number : 755
Status : Valid

| Available Reg Filing : BR |
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : Reviewed
Rev. Date : 2012-11-29
Pay. Date : 2012-11-13
DMF Number : 8656
Submission : 1990-07-11
Status : Active
Type : II

Certificate Number : CEP 2001-352 - Rev 09
Issue Date : 2025-07-03
Type : Chemical
Substance Number : 755
Status : Valid

| Available Reg Filing : CA, ASMF |

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
GDUFA
DMF Review : Complete
Rev. Date : 2013-07-11
Pay. Date : 2013-06-17
DMF Number : 9231
Submission : 1991-07-11
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2013-11-20
Pay. Date : 2013-05-10
DMF Number : 26416
Submission : 2012-09-14
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : Complete
Rev. Date : 2012-11-29
Pay. Date : 2012-11-13
DMF Number : 8296
Submission : 1989-11-14
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : Complete
Rev. Date : 2012-11-29
Pay. Date : 2012-11-13
DMF Number : 8656
Submission : 1990-07-11
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35521
Submission : 2020-12-31
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7361
Submission : 1988-02-29
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8127
Submission : 1989-07-05
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9061
Submission : 1991-04-15
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 9433
Submission : 1991-11-27
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7063
Submission : 1987-06-22
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Parkinson Disease.
Lead Product(s): Levodopa,Carbidopa
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 14, 2016
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 14, 2016
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Parkinson Disease.
Lead Product(s): Levodopa,Carbidopa
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 15, 2015
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 15, 2015
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Parkinson Disease.
Lead Product(s): Levodopa,Carbidopa
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 11, 2013
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
October 11, 2013
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Parkinson Disease.
Lead Product(s): Levodopa,Carbidopa
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 29, 2012
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 29, 2012
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Parkinson Disease.
Lead Product(s): Levodopa,Carbidopa
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Quintiles Inc
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 05, 2011
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 05, 2011
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ABT-SLV187 is a drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Parkinson Disease.
Lead Product(s): ABT-SLV187,Levodopa,Carbidopa
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Undisclosed
Sponsor: Abbott Laboratories
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 24, 2011
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : ABT-SLV187,Levodopa,Carbidopa
Therapeutic Area : Neurology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Abbott Laboratories
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : ABT-SLV187 is a drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Undisclosed
Upfront Cash : Inapplicable
November 24, 2011
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Parkinson Disease.
Lead Product(s): Levodopa,Carbidopa,Neluxicapone
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 04, 2017
Pharmacokinetic of Levodopa Study in Healthy Males
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 04, 2017
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Lead Product(s): Levodopa,Carbidopa,ODM-104
Therapeutic Area: Undisclosed Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 16, 2017
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Levodopa,Carbidopa,ODM-104
Therapeutic Area : Undisclosed
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Dose-finding Pharmacokinetic Study in Healthy Males
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of undefined medical condition.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
February 16, 2017
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Parkinson Disease.
Lead Product(s): Levodopa,Carbidopa,Neluxicapone
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 18, 2015
Pharmacokinetic Study in Healthy Volunteers
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
September 18, 2015
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Parkinson Disease.
Lead Product(s): Levodopa,Carbidopa,Neluxicapone
Therapeutic Area: Neurology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 09, 2014
Pharmacokinetic Study in Healthy Males
Details : Levodopa is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Parkinson Disease.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 09, 2014
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : DUOPA
Dosage Form : SUSPENSION;ENTERAL
Dosage Strength : 4.63MG/ML;20MG/ML
Packaging :
Approval Date : 2015-01-09
Application Number : 203952
Regulatory Info : RX
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Duodopa
Dosage Form : Levodopa+Carbidopa 20Mg/Ml + 5Mg/Ml 100Ml 7 Units' Intestinal Use
Dosage Strength : 7 bags intestinal gel 100 ml 20 mg/ml + 5 mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Karbidopa (Monohydrat); Levodopa
Brand Name : Duodopa
Dosage Form : Gel
Dosage Strength : 20mg/ml;5mg/ml
Packaging :
Approval Date : 21/01/2004
Application Number : 20040121000019
Regulatory Info : Approved
Registration Country : Sweden
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Duodopa
Dosage Form : Gel
Dosage Strength :
Packaging :
Approval Date : 21/12/2006
Application Number : 57624
Regulatory Info : Allowed
Registration Country : Switzerland
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Withdrawn
Registration Country : Malta
Brand Name : Duodopa Gel
Dosage Form : Gel
Dosage Strength : 5MG/ML; 20MG/ML
Packaging :
Approval Date : 2005-10-14
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Duodopa
Dosage Form : Enteral Suspension; Enteral Suspension
Dosage Strength : 20MG/1ML; 4.63MG/1ML
Packaging : 7
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Duodopa
Dosage Form : Enteral Suspension; Enteral Suspension
Dosage Strength : 20MG/1ML; 4.63MG/1ML
Packaging : 7
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Duodopa
Dosage Form : Enteral Suspension; Enteral Suspension
Dosage Strength : 20MG/1ML; 4.63MG/1ML
Packaging : 7
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Duodopa
Dosage Form : Enteral Suspension; Enteral Suspension
Dosage Strength : 20MG/1ML; 4.63MG/1ML
Packaging : 7
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Duodopa
Dosage Form : Enteral Suspension; Enteral Suspension
Dosage Strength : 20MG/1ML; 4.63MG/1ML
Packaging : 7
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : DUOPA
Dosage Form : SUSPENSION;ENTERAL
Dosage Strength : 4.63MG/ML;20MG/ML
Approval Date : 2015-01-09
Application Number : 203952
RX/OTC/DISCN : RX
RLD : Yes
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
CARBIDOPA; ENTACAPONE; LEVODOPA
Brand Name : STALEVO 50
Dosage Form : TABLET;ORAL
Dosage Strength : 12.5MG;200MG;50MG
Approval Date : 2003-06-11
Application Number : 21485
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
CARBIDOPA; ENTACAPONE; LEVODOPA
Brand Name : STALEVO 100
Dosage Form : TABLET;ORAL
Dosage Strength : 25MG;200MG;100MG
Approval Date : 2003-06-11
Application Number : 21485
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
CARBIDOPA; ENTACAPONE; LEVODOPA
Brand Name : STALEVO 150
Dosage Form : TABLET;ORAL
Dosage Strength : 37.5MG;200MG;150MG
Approval Date : 2003-06-11
Application Number : 21485
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
CARBIDOPA; ENTACAPONE; LEVODOPA
Brand Name : STALEVO 200
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG;200MG;200MG
Approval Date : 2007-08-02
Application Number : 21485
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
CARBIDOPA; ENTACAPONE; LEVODOPA
Brand Name : STALEVO 75
Dosage Form : TABLET;ORAL
Dosage Strength : 18.75MG;200MG;75MG
Approval Date : 2008-08-29
Application Number : 21485
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code : AB
CARBIDOPA; ENTACAPONE; LEVODOPA
Brand Name : STALEVO 125
Dosage Form : TABLET;ORAL
Dosage Strength : 31.25MG;200MG;125MG
Approval Date : 2008-08-29
Application Number : 21485
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AB
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AB
Brand Name : CARBIDOPA AND LEVODOPA
Dosage Form : TABLET;ORAL
Dosage Strength : 25MG;100MG
Approval Date : 1992-08-28
Application Number : 73589
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AB
Brand Name : CARBIDOPA AND LEVODOPA
Dosage Form : TABLET;ORAL
Dosage Strength : 25MG;250MG
Approval Date : 1992-08-28
Application Number : 73607
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AB
Brand Name : CARBIDOPA AND LEVODOPA
Dosage Form : TABLET;ORAL
Dosage Strength : 10MG;100MG
Approval Date : 1992-08-28
Application Number : 73618
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Application : Fillers, Diluents & Binders
Excipient Details : It is used as an adhesive agent.
Pharmacopoeia Ref : Not Available
Technical Specs : Also Available as K17, K25, K90 and VA-64
Ingredient(s) : Povidone K30
Application : API Stability Enhancers, Thickeners and Stabilizers
Excipient Details : Adsorbent, Moisture Protection, Stabilization of API
Pharmacopoeia Ref : USP-NF, JP, EP
Technical Specs : Also Available as FUJISIL-F
Ingredient(s) : Silicon Dioxide
Dosage Form : Tablet, Capsule, Granule / Pellet, Injectable / Parenteral
Grade : Oral, Topical
Category : API Stability Enhancers, Fillers, Diluents & Binders, Lubricants & Glidants, Solubilizers
Grade : Not Available
Category : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Application : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Excipient Details : Controlled Release, Direct Compression,Wet Granulation,Tablet Coating, Liquid Solutions and Suspensions
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule
Grade : Oral and Inhalation
Category : API Stability Enhancers, Vegetarian Capsules
Application : API Stability Enhancers, Vegetarian Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : "Water content – less than 9%, can be customized; Size # 00el -...
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule
Grade : Oral
Category : API Stability Enhancers, Taste Masking, Vegetarian Capsules
Application : API Stability Enhancers, Taste Masking, Vegetarian Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : Size # 0-1
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule, Tablet, Dry Syrup
Grade : Oral
Category : Co-Processed Excipients, Direct Compression, Solubilizers
Application : Co-Processed Excipients, Direct Compression, Solubilizers
Excipient Details : Solubilizer
Pharmacopoeia Ref : Not Available
Technical Specs : Solubilizer in powder form; EXCiPACT
Ingredient(s) : Polysorbate 80
Grade : Oral
Category : API Stability Enhancers, Direct Compression, Solubilizers
Application : API Stability Enhancers, Direct Compression, Solubilizers
Excipient Details : Polysorbate 80 in dry powder form, a solubilizing agent acts as a surfactant and increases the solubility of various oral dosage forms.
Pharmacopoeia Ref : USP-NF, EP, JP & having US DMF
Technical Specs : Solubilizer in powder form, used in directly compressible dosage ...
Ingredient(s) : Magnesium aluminium silicate Excipient
https://www.pharmacompass.com/radio-compass-blog/fda-opoe
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
11
PharmaCompass offers a list of Carbidopa API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Carbidopa manufacturer or Carbidopa supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Carbidopa manufacturer or Carbidopa supplier.
PharmaCompass also assists you with knowing the Carbidopa API Price utilized in the formulation of products. Carbidopa API Price is not always fixed or binding as the Carbidopa Price is obtained through a variety of data sources. The Carbidopa Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A NCGC00024596-07 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of NCGC00024596-07, including repackagers and relabelers. The FDA regulates NCGC00024596-07 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. NCGC00024596-07 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of NCGC00024596-07 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A NCGC00024596-07 supplier is an individual or a company that provides NCGC00024596-07 active pharmaceutical ingredient (API) or NCGC00024596-07 finished formulations upon request. The NCGC00024596-07 suppliers may include NCGC00024596-07 API manufacturers, exporters, distributors and traders.
click here to find a list of NCGC00024596-07 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A NCGC00024596-07 DMF (Drug Master File) is a document detailing the whole manufacturing process of NCGC00024596-07 active pharmaceutical ingredient (API) in detail. Different forms of NCGC00024596-07 DMFs exist exist since differing nations have different regulations, such as NCGC00024596-07 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A NCGC00024596-07 DMF submitted to regulatory agencies in the US is known as a USDMF. NCGC00024596-07 USDMF includes data on NCGC00024596-07's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The NCGC00024596-07 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of NCGC00024596-07 suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The NCGC00024596-07 Drug Master File in Japan (NCGC00024596-07 JDMF) empowers NCGC00024596-07 API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the NCGC00024596-07 JDMF during the approval evaluation for pharmaceutical products. At the time of NCGC00024596-07 JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of NCGC00024596-07 suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a NCGC00024596-07 Drug Master File in Korea (NCGC00024596-07 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of NCGC00024596-07. The MFDS reviews the NCGC00024596-07 KDMF as part of the drug registration process and uses the information provided in the NCGC00024596-07 KDMF to evaluate the safety and efficacy of the drug.
After submitting a NCGC00024596-07 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their NCGC00024596-07 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of NCGC00024596-07 suppliers with KDMF on PharmaCompass.
A NCGC00024596-07 CEP of the European Pharmacopoeia monograph is often referred to as a NCGC00024596-07 Certificate of Suitability (COS). The purpose of a NCGC00024596-07 CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of NCGC00024596-07 EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of NCGC00024596-07 to their clients by showing that a NCGC00024596-07 CEP has been issued for it. The manufacturer submits a NCGC00024596-07 CEP (COS) as part of the market authorization procedure, and it takes on the role of a NCGC00024596-07 CEP holder for the record. Additionally, the data presented in the NCGC00024596-07 CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the NCGC00024596-07 DMF.
A NCGC00024596-07 CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. NCGC00024596-07 CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of NCGC00024596-07 suppliers with CEP (COS) on PharmaCompass.
A NCGC00024596-07 written confirmation (NCGC00024596-07 WC) is an official document issued by a regulatory agency to a NCGC00024596-07 manufacturer, verifying that the manufacturing facility of a NCGC00024596-07 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting NCGC00024596-07 APIs or NCGC00024596-07 finished pharmaceutical products to another nation, regulatory agencies frequently require a NCGC00024596-07 WC (written confirmation) as part of the regulatory process.
click here to find a list of NCGC00024596-07 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing NCGC00024596-07 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for NCGC00024596-07 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture NCGC00024596-07 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain NCGC00024596-07 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a NCGC00024596-07 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of NCGC00024596-07 suppliers with NDC on PharmaCompass.
NCGC00024596-07 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of NCGC00024596-07 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right NCGC00024596-07 GMP manufacturer or NCGC00024596-07 GMP API supplier for your needs.
A NCGC00024596-07 CoA (Certificate of Analysis) is a formal document that attests to NCGC00024596-07's compliance with NCGC00024596-07 specifications and serves as a tool for batch-level quality control.
NCGC00024596-07 CoA mostly includes findings from lab analyses of a specific batch. For each NCGC00024596-07 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
NCGC00024596-07 may be tested according to a variety of international standards, such as European Pharmacopoeia (NCGC00024596-07 EP), NCGC00024596-07 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (NCGC00024596-07 USP).

