07 Aug 2025
// PRESS RELEASE
17 Jun 2025
// PRESS RELEASE
23 Apr 2025
// PRESS RELEASE
 KEY PRODUCTS
KEY PRODUCTS KEY SERVICES
KEY SERVICES
With Fermion, start the journey of your innovative API.
About
Asia Pharma ExpoAsia Pharma Expo
Industry Trade Show
Not Confirmed
12-14 February, 2026
Industry Trade Show
Not Confirmed
14-17 February, 2026
Suppliers Night ExpoSuppliers Night Expo
Industry Trade Show
Not Confirmed
16-17 February, 2026
CONTACT DETAILS





Events
Webinars & Exhibitions
Asia Pharma ExpoAsia Pharma Expo
Industry Trade Show
Not Confirmed
12-14 February, 2026
Industry Trade Show
Not Confirmed
14-17 February, 2026
Suppliers Night ExpoSuppliers Night Expo
Industry Trade Show
Not Confirmed
16-17 February, 2026
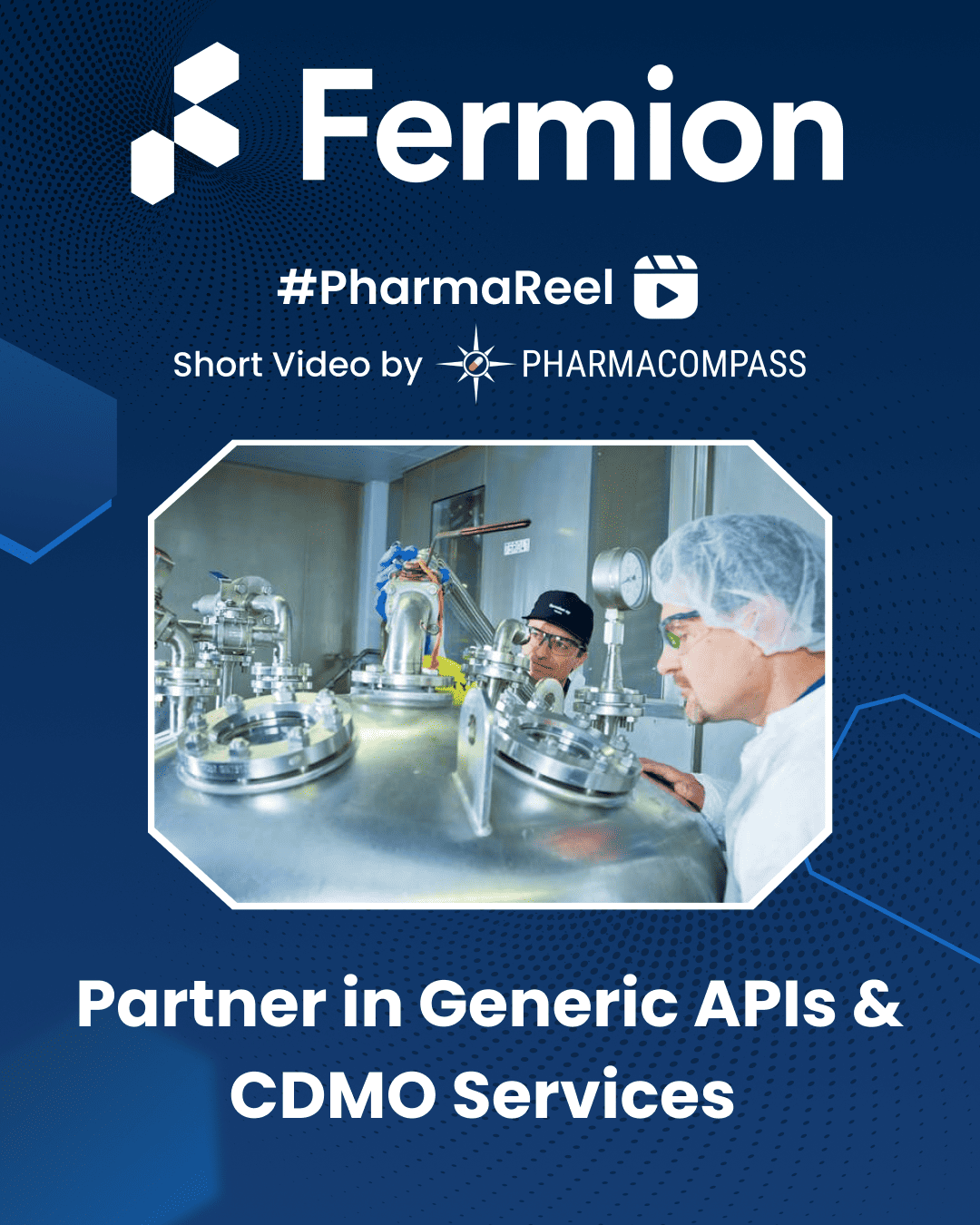
VLOG #PharmaReel
CORPORATE CONTENT #SupplierSpotlight
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-veranova-chemexpress-invest-in-adc-facilities-cohance-to-set-up-oligonucleotide-facility-in-india
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-veranova-carbogen-lead-adc-investments-axplora-polfa-tarchomin-famar-expand-european-footprint
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-bora-polpharma-make-acquisitions-evonik-euroapi-porton-announce-technological-expansions
https://www.pharmacompass.com/radio-compass-blog/cdmo-activity-tracker-novo-s-parent-buys-catalent-for-us-16-5-bn-fujifilm-merck-kgaa-axplora-lonza-expand-capabilities

07 Aug 2025
// PRESS RELEASE
https://www.fermion.fi/who-we-are/news/fermion-begins-renovation-to-expand-api-manufacturing-capacity-at-hanko-site/

17 Jun 2025
// PRESS RELEASE
https://www.fermion.fi/who-we-are/news/crystallization-as-a-core-competence-in-api-manufacturing/

23 Apr 2025
// PRESS RELEASE
https://www.fermion.fi/who-we-are/news/new-micronization-equipment-at-fermion-oulu-plant/

02 Jan 2025
// PRESS RELEASE
https://www.fermion.fi/who-we-are/news/fermion-invests-in-flow-chemistry-capability/

29 Oct 2024
// PRESS RELEASE
https://www.fermion.fi/who-we-are/news/fermion---leader-in-sustainable-api-manufacturing/#:~:text=Sustainability%20is%20an%20integral%20part,Fermion%20plays%20an%20important%20role.

20 Aug 2024
// PRESS RELEASE
Services
Analytical
API Manufacturing
Drug Product Manufacturing
API & Drug Product Development
Packaging
ABOUT THIS PAGE
Fermion Oy is a supplier offers 41 products (APIs, Excipients or Intermediates).
Find a price of Alprazolam bulk with DMF, CEP, JDMF offered by Fermion Oy
Find a price of Azathioprine bulk with DMF, CEP, JDMF offered by Fermion Oy
Find a price of Formoterol Fumarate bulk with DMF, CEP, JDMF offered by Fermion Oy
Find a price of Hydroxychloroquine Sulphate bulk with DMF, CEP, JDMF offered by Fermion Oy
Find a price of Irinotecan Hydrochloride bulk with DMF, CEP, JDMF offered by Fermion Oy
Find a price of Mercaptopurine bulk with DMF, CEP, JDMF offered by Fermion Oy
Find a price of Methotrexate bulk with DMF, CEP, JDMF offered by Fermion Oy
Find a price of Sodium Cromoglicate bulk with DMF, CEP, JDMF offered by Fermion Oy
Find a price of Buspirone Hydrochloride bulk with DMF, CEP offered by Fermion Oy
Find a price of Carbidopa bulk with DMF, CEP offered by Fermion Oy
Find a price of Dexmedetomidine Hydrochloride bulk with DMF, JDMF offered by Fermion Oy
Find a price of Entacapone bulk with DMF, CEP offered by Fermion Oy
Find a price of Fluoxetine Hydrochloride bulk with DMF, CEP offered by Fermion Oy
Find a price of Flutamide bulk with DMF, CEP offered by Fermion Oy
Find a price of Nadolol bulk with DMF, JDMF offered by Fermion Oy
Find a price of Propafenone Hydrochloride bulk with DMF, CEP offered by Fermion Oy
Find a price of Quetiapine Hemifumarate bulk with DMF, CEP offered by Fermion Oy
Find a price of Toremifene Citrate bulk with DMF, JDMF offered by Fermion Oy
Find a price of Benserazide Hydrochloride bulk with CEP offered by Fermion Oy
Find a price of Budesonide bulk with CEP offered by Fermion Oy
Find a price of Carbidopa bulk with CEP offered by Fermion Oy
Find a price of Diltiazem Hydrochloride bulk with DMF offered by Fermion Oy
Find a price of Glipizide bulk with DMF offered by Fermion Oy
Find a price of Levosimendan bulk with DMF offered by Fermion Oy
Find a price of Methotrexate bulk with DMF offered by Fermion Oy
Find a price of Nintedanib Esylate bulk with DMF offered by Fermion Oy
Find a price of Ospemifene bulk with DMF offered by Fermion Oy
Find a price of Quetiapine Hemifumarate bulk with DMF offered by Fermion Oy
Find a price of Salmeterol Xinafoate bulk with CEP offered by Fermion Oy
Find a price of Tamsulosin bulk with JDMF offered by Fermion Oy
Find a price of Tolnaftate bulk with DMF offered by Fermion Oy
Find a price of Treosulfan bulk with DMF offered by Fermion Oy
Find a price of Atipamezole Hydrochloride bulk offered by Fermion Oy
Find a price of Dabrafenib Mesylate bulk offered by Fermion Oy
Find a price of Darolutamide bulk offered by Fermion Oy
Find a price of Detomidine bulk offered by Fermion Oy
Find a price of Dexmedetomidine Hydrochloride bulk offered by Fermion Oy
Find a price of Osimertinib Mesylate bulk offered by Fermion Oy
Find a price of Rucaparib Camsylate bulk offered by Fermion Oy
Find a price of Trilaciclib Dihydrochloride bulk offered by Fermion Oy
Find a price of Vismodegib bulk offered by Fermion Oy


 Fermion Oy
Fermion Oy