
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
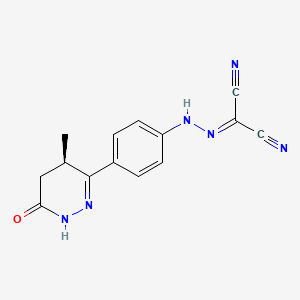

1. ((4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono)propanedinitrile
2. Dextrosimendan
3. Or 1259
4. Or 1855
5. Or-1259
6. Or-1855
7. Simadax
8. Simendan
1. 141505-33-1
2. Simdax
3. (r)-simendan
4. (-)-or-1259
5. Or-1259
6. Simendan, (r)-
7. Chebi:50567
8. 2-[[4-[(4r)-4-methyl-6-oxo-4,5-dihydro-1h-pyridazin-3-yl]phenyl]hydrazinylidene]propanedinitrile
9. Nsc-759644
10. Levosimedan
11. C6t4514l4e
12. Or1259
13. (r)-((4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono) Propanedintrile
14. (r)-n-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)carbonohydrazonoyl Dicyanide
15. Levosimendan [inn]
16. Dsstox_cid_26445
17. Dsstox_rid_81620
18. Mesoxalonitrile (p-((r)-1,4,5,6-tetrahydro-4-methyl-6-oxo-pyridazinyl)phenyl)hydrazone
19. Dsstox_gsid_46445
20. Levosimendanum
21. Smr002529692
22. Simdax (tn)
23. Cas-141505-33-1
24. Levosimendan (usan/inn)
25. Levosimendan [usan:inn]
26. Or 1259
27. Unii-c6t4514l4e
28. ((4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono)propanedinitrile
29. Levosimendan- Bio-x
30. Levosimendan [mi]
31. Levosimendan [usan]
32. Mesoxalonitrile (-)-(p((r)-1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazone
33. Schembl83243
34. Levosimendan [mart.]
35. Mls003899227
36. Mls006010741
37. Levosimendan [who-dd]
38. Chembl2051955
39. Dtxsid9046445
40. Levosimendan, >=98% (hplc)
41. Hms3264g03
42. Hms3884n17
43. Kuc109648n
44. Pharmakon1600-01502356
45. Act02710
46. Bcp07048
47. Zinc3915645
48. Tox21_112191
49. Tox21_113768
50. Bdbm50469700
51. Mfcd00867135
52. Nsc759644
53. S2446
54. Akos015895214
55. Tox21_112191_1
56. Ac-1752
57. Am84381
58. Ccg-213048
59. Db00922
60. Ds-8918
61. Nsc 759644
62. ({4-[(4r)-4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl]phenyl}hydrazono)propanedintrile
63. 2-[[4-[(4r)-4-methyl-6-oxo-4,5-dihydro-1h-pyridazin-3-yl]phenyl]hydrazono]propanedinitrile
64. Ksc-210-010
65. Ncgc00253641-01
66. Ncgc00263564-01
67. Ncgc00263564-02
68. Bm164625
69. Hy-14286
70. L0320
71. Sw219172-1
72. A11874
73. D04720
74. N12889
75. Ab01562970_01
76. Ab01562970_02
77. 741l087
78. A807767
79. Q162541
80. Sr-01000931342
81. Sr-01000931342-2
82. 1-beta-d-ribofuranose-1h-1,2,4-triazole-3-methylcarbonate
83. (r)-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)carbonohydrazonoyl Dicyanide
84. (r)-n-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)carbonohydrazonoyldicyanide
85. 1-cyano-n-{4-[(4r)-4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl]phenyl}methanecarbohydrazonoyl Cyanide
86. 2-(2-(4-((4r)-1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazinylidene)propanedinitrile
87. Mesoxalonitrile (-)-(p((r)-1,4,5,6-tetrahydro-4-methyl-6- Oxo-3-pyridazinyl)phenyl)hydrazone
88. Propanedinitrile, ((4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono)-, (r)-
89. Propanedinitrile, 2-(2-(4-((4r)-1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazinylidene)-
| Molecular Weight | 280.28 g/mol |
|---|---|
| Molecular Formula | C14H12N6O |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 280.10725903 g/mol |
| Monoisotopic Mass | 280.10725903 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 549 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For short term treatment of acutely decompensated severe chronic heart failure (CHF). Also being investigated for use/treatment in heart disease.
Levosimendan is a new Ca2+-sensitizing inotropic agent. Ca2+ sensitizers represent a new class of inotropic agents, which overcome the disadvantages associated with currently available inotropic agents in as they are not associated with an increased risk of arrhythmias, cell injury and death due to Ca2+ overload in myocardial cells; they do not increase the activation energy; and they have the potential to reverse contractile dysfunction under pathophysiologic conditions, such as acidosis or myocardial stunning. Levosimendan has not been approved for use in the U.S. or Canada.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
Phosphodiesterase 3 Inhibitors
Compounds that specifically inhibit PHOSPHODIESTERASE 3. (See all compounds classified as Phosphodiesterase 3 Inhibitors.)
C - Cardiovascular system
C01 - Cardiac therapy
C01C - Cardiac stimulants excl. cardiac glycosides
C01CX - Other cardiac stimulants
C01CX08 - Levosimendan
Absorption
The bioavailability of oral levosimendan is 85 ± 6% in healthy volunteers and 84 ± 4% in patients.
Complete metabolism, with some active metabolites (OR-1855 and OR-1896) possibly extending the drug's haemodynamic effects.
Eliminination half-life is approximately 1 hour.
Levosimendan appears to increase myofilament calcium sensitivity by binding to cardiac troponin C in a calcium-dependent manner. This stabilizes the calcium-induced conformational change of troponin C, thereby (1) changing actin-myosin cross-bridge kinetics apparently without increasing the cycling rate of the cross-bridges or myocardial ATP consumption, (2) increasing the effects of calcium on cardiac myofilaments during systole and (3) improving contraction at low energy cost (inotropic effect). Calcium concentration and, therefore, sensitization decline during diastole, allowing normal or improved diastolic relaxation. Levosimendan also leads to vasodilation through the opening of ATP-sensitive potassium channels. By these inotropic and vasodilatory actions, levosimendan increases cardiac output without increasing myocardial oxygen demand. Levosimendan also has a selective phosphodiesterase (PDE)-III inhibitory action that may contribute to the inotropic effect of this compound under certain experimental conditions. It has been reported that levosimendan may act preferentially as a Ca2+ sensitizer at lower concentrations, whereas at higher concentrations its action as a PDE-III inhibitor becomes more prominent in experimental animals and humans.
 Transo-Pharm GmbH works globally to supply Active Pharmaceutical Ingredients adhering to the highest quality & GMP standards.
Transo-Pharm GmbH works globally to supply Active Pharmaceutical Ingredients adhering to the highest quality & GMP standards.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12960
Submission : 1998-04-24
Status : Active
Type : II

| Available Reg Filing : ASMF |

Date of Issue : 2024-04-16
Valid Till : 2027-05-21
Written Confirmation Number : SD240029
Address of the Firm :
 Reliable Spanish CDMO Delivering High-Quality APIs and Intermediates with Excellence, Flexibility, and Regulatory Compliance.
Reliable Spanish CDMO Delivering High-Quality APIs and Intermediates with Excellence, Flexibility, and Regulatory Compliance.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 37514
Submission : 2022-09-29
Status : Active
Type : II

Date of Issue : 2025-10-24
Valid Till : 2028-02-07
Written Confirmation Number : WC-0227
Address of the Firm :



 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
42
PharmaCompass offers a list of Levosimendan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Levosimendan manufacturer or Levosimendan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Levosimendan manufacturer or Levosimendan supplier.
PharmaCompass also assists you with knowing the Levosimendan API Price utilized in the formulation of products. Levosimendan API Price is not always fixed or binding as the Levosimendan Price is obtained through a variety of data sources. The Levosimendan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Levosimendan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Levosimendan, including repackagers and relabelers. The FDA regulates Levosimendan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Levosimendan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Levosimendan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Levosimendan supplier is an individual or a company that provides Levosimendan active pharmaceutical ingredient (API) or Levosimendan finished formulations upon request. The Levosimendan suppliers may include Levosimendan API manufacturers, exporters, distributors and traders.
click here to find a list of Levosimendan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Levosimendan DMF (Drug Master File) is a document detailing the whole manufacturing process of Levosimendan active pharmaceutical ingredient (API) in detail. Different forms of Levosimendan DMFs exist exist since differing nations have different regulations, such as Levosimendan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Levosimendan DMF submitted to regulatory agencies in the US is known as a USDMF. Levosimendan USDMF includes data on Levosimendan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Levosimendan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Levosimendan suppliers with USDMF on PharmaCompass.
A Levosimendan written confirmation (Levosimendan WC) is an official document issued by a regulatory agency to a Levosimendan manufacturer, verifying that the manufacturing facility of a Levosimendan active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Levosimendan APIs or Levosimendan finished pharmaceutical products to another nation, regulatory agencies frequently require a Levosimendan WC (written confirmation) as part of the regulatory process.
click here to find a list of Levosimendan suppliers with Written Confirmation (WC) on PharmaCompass.
Levosimendan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Levosimendan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Levosimendan GMP manufacturer or Levosimendan GMP API supplier for your needs.
A Levosimendan CoA (Certificate of Analysis) is a formal document that attests to Levosimendan's compliance with Levosimendan specifications and serves as a tool for batch-level quality control.
Levosimendan CoA mostly includes findings from lab analyses of a specific batch. For each Levosimendan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Levosimendan may be tested according to a variety of international standards, such as European Pharmacopoeia (Levosimendan EP), Levosimendan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Levosimendan USP).

