
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
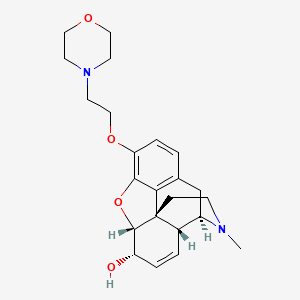

1. 7,8-didehydro-4,5 Alpha-epoxy-17-methyl-3-(2-morpholinoethoxymorphinan-6 Alpha-ol)
2. Homocodeine
1. Homocodeine
2. Morpholinylethylmorphine
3. Folcodine
4. Glycodine
5. Neocodine
6. Weifacodine
7. Codylin
8. Pholtex
9. Hibernyl
10. Tussokon
11. Ethnine
12. Memine
13. Pholcodinum
14. Folcodina
15. Galenphol
16. 509-67-1
17. Biocalyptol
18. Pectolin
19. 3-morpholylaethylmorphin
20. Beta-morpholinoethylmorphine
21. Folkodin
22. Neocodin
23. Pholcodin
24. Ethnine Simplex
25. Dia-tuss
26. 3-(2-(4-morpholinyl)ethyl)morphine
27. Tetrahydro-1,4-oxazinylmethylcodeine
28. Galphol
29. 3-(2-morpholinoethyl)morphine
30. Dea No. 9314
31. Chebi:53579
32. 3-morpholinoethylmorphine
33. Lpp64awz7l
34. 7,8-didehydro-4,5-alpha-epoxy-17-methyl-3-(2-morpholinoethoxy)morphinan-6-alpha-ol
35. Pholcodine (inn)
36. Pholcodine [inn]
37. (5alpha,6alpha)-17-methyl-3-[2-(morpholin-4-yl)ethoxy]-7,8-didehydro-4,5-epoxymorphinan-6-ol
38. Prodromine
39. O3-(2-morpholinoethyl)morphine
40. Morphine, 3-o-(2-morpholinoethyl)-
41. Folkodin [czech]
42. (4r,4ar,7s,7ar,12bs)-3-methyl-9-(2-morpholin-4-ylethoxy)-2,4,4a,7,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinolin-7-ol
43. Folcodina [spanish]
44. Pholcodinum [latin]
45. Pholcodine [inn:ban]
46. Folcodina [inn-spanish]
47. Pholcodinum [inn-latin]
48. Unii-lpp64awz7l
49. Beta-morpholinylethylmorphine
50. Adaphol
51. Codisol
52. Copholco
53. Lantuss
54. Pholcolin
55. Pavacol-d
56. 3-morpholylaethylmorphin [german]
57. Covonia Dry Cough
58. Einecs 208-102-9
59. O(sup 3)-(2-morpholinoethyl)morphine
60. 3-morpholylathylmorphin
61. Pholcodine [mi]
62. Morphine, O(sup 3)-(2-morpholinoethyl)-
63. Pholcodine Linctus (tn)
64. Epitope Id:116651
65. Pholcodine [who-dd]
66. Schembl154354
67. Gtpl9086
68. Chembl2105224
69. Dtxsid70198923
70. 03-(2-morpholinoethyl)-morphine
71. Ids-np-011(sect.2)
72. 3-o-(2-morpholinoethyl)-morphine
73. Morphinan-6-ol, 7,8-didehydro-4,5-epoxy-17-methyl-3-(2-(4-morpholinyl)ethoxy)-, (5.alpha.,6.alpha.)-
74. Zinc4217287
75. Db09209
76. D07385
77. Q3124290
78. Morphinan-6-alpha-ol, 7,8-didehydro-4,5-alpha-epoxy-17-methyl-3-(2-morpholinoethoxy)-
79. (1s,5r,13r,14s,17r)-4-methyl-10-[2-(morpholin-4-yl)ethoxy]-12-oxa-4-azapentacyclo[9.6.1.0^{1,13}.0^{5,17}.0^{7,18}]octadeca-7(18),8,10,15-tetraen-14-ol
80. (4r,4ar,7s,7ar,12bs)-3-methyl-9-(2-morpholin-4-ylethoxy)-2,4,4a,7,7a,13-hexahydro-1h-4,12-methanobenzofuro[3,2-e]isoquinoline-7-o
| Molecular Weight | 398.5 g/mol |
|---|---|
| Molecular Formula | C23H30N2O4 |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 398.22055744 g/mol |
| Monoisotopic Mass | 398.22055744 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 653 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Pholcodine is indicated as a cough suppressant for the temporary relief of non-productive dry cough. It is stated to present a required label indication of "temporary relief of dry cough." Cough is the respiratory movement that occurs after an irritation signal is transmitted to the central nervous system and further stimulates the medulla oblongata. This stimulation causes a motor output that is sent through motoneurons to the respiratory muscles. A non-productive cough is a type of cough characterized by the absence of sputum, and it has a large inspiration that will cause continuous coughing.
The therapeutic doses of pholcodine have been shown not to cause depression of respiration, CNS excitation or other side effects associated with narcotics. It is thought that the impact of pholcodine is selective on the cough center without affecting the respiratory center. Pholcodine is not euphorigenic, and thus, psychological dependence is unlikely. Clinical trials have not shown any evidence of addiction after prolonged administration of pholcodine. It is well reported that pholcodine presents a more considerable respiratory depression effect than codeine and it causes hypotension in the same degree than codeine. Some other noted impacts of pholcodine in preclinical trials are: 1) the induction of histamine release, 2) anti-histaminic effect, 3) anti-acetylcholinic action, 4) anti-convulsant action and 5) mild tranquilizing action.
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
R - Respiratory system
R05 - Cough and cold preparations
R05D - Cough suppressants, excl. combinations with expectorants
R05DA - Opium alkaloids and derivatives
R05DA08 - Pholcodine
Absorption
After oral administration of 60 mg of pholcodine, the Tmax and Cmax are reported to be 1.3 hours and 26.3 ng/ml. In the same administration, the AUC in plasma and saliva are reported to be 1.67 and 6.61 mg h/l respectively. The absorption of pholcodine is reported to represent approximately 88% of the administered dose.
Route of Elimination
After oral administration of pholcodine, the serum concentration peaks and declines in a monoexponential manner. The percent of the dose excreted unchanged is of approximately 25-30%. Part of the administered dose is composed by metabolites that can be recovered in urine. From the administered dose, the fecal excretion corresponds to the 5% of the administered dose as unchanged pholcodine.
Volume of Distribution
The reported volume of distribution depends on the pharmacokinetic model and it can be of 265L based on a one-compartment model to 3207L in a two-compartment model.
Clearance
After oral administration of 60 mg of pholcodine, the clearance rate was reported to be 126 ml/min.
The metabolism of pholcodine seems to be very slow and due to the elimination profile, it is thought that most of the administered dose undergoes metabolism. There is some evidence in preclinical trials that indicate that morphine is a minor metabolite of pholcodine and that it accounts for 1% of the administered dose.
After oral administration of 60 mg of pholcodine, the half-life in plasma, saliva and urine are 45, 55 and 45 hours respectively.
The mechanism of action of pholcodine is directly performed in the medulla oblongata. In this site, it exerts analgesic properties on the peripheric reflexogenic receptors. This site is commonly known as the "cough center."

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
18
PharmaCompass offers a list of Pholcodine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pholcodine manufacturer or Pholcodine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pholcodine manufacturer or Pholcodine supplier.
PharmaCompass also assists you with knowing the Pholcodine API Price utilized in the formulation of products. Pholcodine API Price is not always fixed or binding as the Pholcodine Price is obtained through a variety of data sources. The Pholcodine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Folcodin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Folcodin, including repackagers and relabelers. The FDA regulates Folcodin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Folcodin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Folcodin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Folcodin supplier is an individual or a company that provides Folcodin active pharmaceutical ingredient (API) or Folcodin finished formulations upon request. The Folcodin suppliers may include Folcodin API manufacturers, exporters, distributors and traders.
click here to find a list of Folcodin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Folcodin DMF (Drug Master File) is a document detailing the whole manufacturing process of Folcodin active pharmaceutical ingredient (API) in detail. Different forms of Folcodin DMFs exist exist since differing nations have different regulations, such as Folcodin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Folcodin DMF submitted to regulatory agencies in the US is known as a USDMF. Folcodin USDMF includes data on Folcodin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Folcodin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Folcodin suppliers with USDMF on PharmaCompass.
A Folcodin CEP of the European Pharmacopoeia monograph is often referred to as a Folcodin Certificate of Suitability (COS). The purpose of a Folcodin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Folcodin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Folcodin to their clients by showing that a Folcodin CEP has been issued for it. The manufacturer submits a Folcodin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Folcodin CEP holder for the record. Additionally, the data presented in the Folcodin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Folcodin DMF.
A Folcodin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Folcodin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Folcodin suppliers with CEP (COS) on PharmaCompass.
Folcodin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Folcodin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Folcodin GMP manufacturer or Folcodin GMP API supplier for your needs.
A Folcodin CoA (Certificate of Analysis) is a formal document that attests to Folcodin's compliance with Folcodin specifications and serves as a tool for batch-level quality control.
Folcodin CoA mostly includes findings from lab analyses of a specific batch. For each Folcodin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Folcodin may be tested according to a variety of international standards, such as European Pharmacopoeia (Folcodin EP), Folcodin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Folcodin USP).

