
Synopsis
Synopsis
0
VMF
0
Australia
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
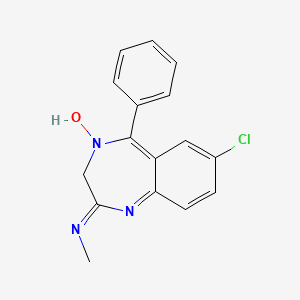

1. 7 Chloro 2 Methylamino 5 Phenyl 3h 1,4 Benzodiazepine 4 Oxide
2. 7 Chloro N Methyl 5 Phenyl 3h 1,4 Benzodiazepin 2 Amine 4 Oxide
3. 7-chloro-2-methylamino-5-phenyl-3h-1,4-benzodiazepine-4-oxide
4. 7-chloro-n-methyl-5-phenyl-3h-1,4-benzodiazepin-2-amine 4-oxide
5. Chlordiazepoxide Hydrobromide
6. Chlordiazepoxide Hydrochloride
7. Chlordiazepoxide Monohydrochloride
8. Chlordiazepoxide Perchlorate
9. Chlozepid
10. Elenium
11. Hydrobromide, Chlordiazepoxide
12. Hydrochloride, Chlordiazepoxide
13. Librium
14. Methaminodiazepoxide
15. Monohydrochloride, Chlordiazepoxide
16. Perchlorate, Chlordiazepoxide
1. 58-25-3
2. Methaminodiazepoxide
3. Librium
4. Chlordiazepoxid
5. Librelease
6. Libritabs
7. Clopoxide
8. Radepur
9. Lygen
10. 7-chloro-n-methyl-5-phenyl-3h-1,4-benzodiazepin-2-amine 4-oxide
11. 3h-1,4-benzodiazepin-2-amine, 7-chloro-n-methyl-5-phenyl-, 4-oxide
12. 7-chloro-4-hydroxy-n-methyl-5-phenyl-3h-1,4-benzodiazepin-2-imine
13. Chlordiazepoxide Civ
14. Chlozepid
15. Elenium
16. Chlorodiazepoxide
17. Chloridiazepide
18. Helogaphen
19. Kalmocaps
20. Viopsicol
21. Control
22. Decacil
23. Ifibrium
24. Librinin
25. Menrium
26. Mesural
27. Mildmen
28. Napoton
29. Psicosan
30. Risolid
31. Silibrin
32. Tropium
33. 6rz6xez3cr
34. Zetran
35. Chembl451
36. 7-chloro-2-(methylamino)-5-phenyl-3h-1,4-benzodiazepine 4-oxide
37. Disarim
38. Sonimen
39. Zeisin
40. Clordiazepossido
41. Eden-psich
42. Chlordiazepoxidum
43. Chlordiazepoxydum
44. 3h-1,4-benzodiazepine, 7-chloro-2-(methylamino)-5-phenyl-, 4-oxide
45. Clordiazepoxido
46. Chloradiazepoxide
47. Balance (pharmaceutical)
48. Dsstox_cid_26022
49. Dsstox_rid_81295
50. Dsstox_gsid_46022
51. Clordiazepossido [italian]
52. Chlordiazepoxidum [inn-latin]
53. Clordiazepoxido [inn-spanish]
54. 7-chloro-2-methylamino-5-phenyl-3h-1,4-benzodiazepin-4-oxide
55. Cas-58-25-3
56. Libritabs (tn)
57. Smr000469226
58. Hsdb 3028
59. Einecs 200-371-0
60. Unii-6rz6xez3cr
61. 7-chloro-n-methyl-4-oxido-5-phenyl-3h-1,4-benzodiazepin-4-ium-2-amine
62. Chlordiazepoxide-hcl
63. 7-chlor-2-methylamino-5-phenyl-3h-1,4-benzodiazepin-4-oxid [german]
64. 7-cloro-2-metilamino-5-fenil-3h-1,4-benzodiazepina 4-ossido [italian]
65. Chlordiazepoxide [usp:inn:ban:jan]
66. Chlordiazep-oxide Hcl
67. 7-chlor-2-methylamino-5-phenyl-3h-1,4-benzodiazepin-4-oxid
68. 7-cloro-2-metilamino-5-fenil-3h-1,4-benzodiazepina 4-ossido
69. Spectrum2_001157
70. Spectrum4_000578
71. Spectrum5_001623
72. Schembl18474
73. Chlordiazepoxide [mi]
74. Kbiogr_001016
75. Mls001066622
76. Mls001424220
77. Mls003899195
78. Chlordiazepoxide [inn]
79. Chlordiazepoxide [jan]
80. Divk1c_000995
81. Schembl145243
82. Spbio_001113
83. Chlordiazepoxide [hsdb]
84. Chebi:3611
85. Gtpl3370
86. Chlordiazepoxide [vandf]
87. Chlordiazepoxide [mart.]
88. Chebi:94781
89. Hms503g11
90. Kbio1_000995
91. Chlordiazepoxide [who-dd]
92. Dtxsid40861935
93. Ninds_000995
94. Hms2052c19
95. Hms2231m10
96. Hms3373m10
97. Hms3394c19
98. 7-chloro-2-methylamino-5-phenyl-3h-1,4-benzodiazepine-4-oxide
99. Chlordiazepoxide (jp17/usp/inn)
100. Tox21_111567
101. Tox21_111568
102. 3h-1,4-benzodiazepin-2-amine,7-chloro-n-methyl-5-phenyl-,4-oxide
103. Bbl010791
104. Bdbm50007664
105. Stk597142
106. Zinc19632917
107. Chlordiazepoxide [orange Book]
108. Chlordiazepoxide Civ [usp-rs]
109. Akos005518509
110. Akos015963131
111. Ab06298
112. Ccg-101136
113. Chlordiazepoxide [ep Monograph]
114. Db00475
115. Nc00386
116. Chlordiazepoxide [usp Monograph]
117. Chlordiazepoxide 1.0 Mg/ml In Methanol
118. Idi1_000995
119. Menrium Component Chlordiazepoxide
120. Ncgc00246347-01
121. Ac-13012
122. Vs-02683
123. Limbitrol Component Chlordiazepoxide
124. Chlordiazepoxide Component Of Menrium
125. Db-053173
126. Chlordiazepoxide Component Of Limbitrol
127. Limbitrol Ds Component Chlordiazepoxide
128. D00267
129. Ab00053219-06
130. Chlordiazepoxide Component Of Limbitrol Ds
131. Q178566
132. W-105404
133. 7-chloro-2-(methylamino)-5-phenyl-3h-1,4-benzodiazepin-4-ium-4-olate
134. 7-chloro-2-(methylimino)-5-phenyl-2,3-dihydro-4h-1,4-benzodiazepin-4-ol
135. (1e,4e)-7-chloro-2-(methylamino)-5-phenyl-3h-benzo[e][1,4]diazepine 4-oxide
| Molecular Weight | 299.75 g/mol |
|---|---|
| Molecular Formula | C16H14ClN3O |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 299.0825398 g/mol |
| Monoisotopic Mass | 299.0825398 g/mol |
| Topological Polar Surface Area | 48.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 580 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Librium |
| PubMed Health | Chlordiazepoxide (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Librium, the original chlordiazepoxide HCl and prototype for the benzodiazepine compounds, was synthesized and developed at Hoffmann-La Roche Inc. It is a versatile therapeutic agent of proven value for the relief of anxiety. Librium is among the... |
| Active Ingredient | Chlordiazepoxide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 2 of 2 | |
|---|---|
| Drug Name | Librium |
| PubMed Health | Chlordiazepoxide (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Librium, the original chlordiazepoxide HCl and prototype for the benzodiazepine compounds, was synthesized and developed at Hoffmann-La Roche Inc. It is a versatile therapeutic agent of proven value for the relief of anxiety. Librium is among the... |
| Active Ingredient | Chlordiazepoxide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
Adjuvants, Anesthesia; Anti-Anxiety Agents, Benzodiazepine; GABA Modulators; Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): 15-30% LEVEL IN FEED, PRODUCES ... /CNS DEPRESSION/ IN COWBIRDS; QUAILS (5-10% LEVEL) SHOW EXCITEMENT PHASE BEFORE ... /CNS DEPRESSION/. 3-10 MG/KG IP AGAINST AGGRESSIVE BEHAVIOR IN RATS. AS CNS DEPRESSANT (80 MG/KG) IN RATS & MICE. AS ADRENERGIC BLOCKING AGENT (20 MG/KG) IN MICE. IN ZOO ANIMALS, 4-25 MG/KG BY SUITABLE ROUTE OF ADMIN HAS TAMING EFFECT ON VICIOUS ANIMALS; BARBITURATES ARE USED AS ADJUNCTIVES. IN RHESUS MONKEYS AS ANTICONVULSANT (4.4 MG/KG IV) /FROM TABLE/
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 101
Chlordiazepoxide ... is used for ... the management of agitation associated with acute alcohol withdrawal.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1942
... AS PREMEDICATION IN ANESTHESIA & IN OBSTETRICS DURING LABOR. /BENZODIAZEPINES/
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 436
For more Therapeutic Uses (Complete) data for CHLORDIAZEPOXIDE (7 total), please visit the HSDB record page.
CHLORDIAZEPOXIDE HYDROCHLORIDE REQUIRES ... PRECAUTIONS REGARDING ITS USE IN PT WITH KNOWN HYPERSENSITIVITY, ELDERLY & EXCESSIVELY DEPRESSED INDIVIDUALS, PREGNANT & LACTATING MOTHERS, PT WITH KNOWN RENAL & HEPATIC IMPAIRMENT, PT ON OTHER CNS DEPRESSANT DRUGS, & IN PT WITH EITHER A HISTORY OF DRUG ADDICTION OR INDISCRIMINANT ALTERATION OF DRUG DOSAGE. /CHLORDIAZEPOXIDE HYDROCHLORIDE/
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1006
Safety and efficacy of orally administered chlordiazepoxide ... in children younger than 6 years of age ... have not been established.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1942
The iv preparation reconstituted with 0.9% sodium chloride injection or sterile water for injection should not be given im /SRP: absorption from this site is very slow and variable/ injection.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1942
THE EFFECT OF NORMAL ORAL DOSES (10 MG 3 TIMES A DAY) OF CHLORDIAZEPOZIDE (LIBRIUM) WAS STUDIED IN A DOUBLE-BLIND CROSSOVER TRIAL IN 7 PATIENTS WITH RESPIRATORY FAILURE DUE PREDOMINANTLY TO CHRONIC BRONCHITIS. IN 6 PATIENTS THE DRUG CAUSED A HIGHLY SIGNIFICANT INCREASE IN MIXED VENOUS CARBON-DIOXIDE TENSION AND A SIGNIFICANT FALL IN FORCED EXPIRATORY VOLUME IN ONE SECOND. IT IS CONCLUDED THAT CHLORDIAZEPOXIDE IS CONTRAINDICATED IN PATIENTS WITH RESPIRATORY FAILURE DUE TO CHRONIC BRONCHITIS.
PMID:4137638 MODEL DG, BERRY DJ; LANCET 2 (OCT 12): 869-70 (1974)
For more Drug Warnings (Complete) data for CHLORDIAZEPOXIDE (13 total), please visit the HSDB record page.
A few deaths have been reported at doses greater than 700 mg of ... chlordiazepoxide.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 424
For the management of anxiety disorders or for the short-term relief of symptoms of anxiety, withdrawal symptoms of acute alcoholism, and preoperative apprehension and anxiety.
Chlordiazepoxide has antianxiety, sedative, appetite-stimulating and weak analgesic actions. The drug seems to block EEG arousal from stimulation in the brain stem reticular formation. The drug has been studied extensively in many species of animals and these studies are suggestive of action on the limbic system of the brain, which recent evidence indicates is involved in emotional responses. Hostile monkeys were made tame by oral drug doses which did not cause sedation. Chlordiazepoxide revealed a "taming" action with the elimination of fear and aggression. The taming effect of chlordiazepoxide was further demonstrated in rats made vicious by lesions in the septal area of the brain. The drug dosage which effectively blocked the vicious reaction was well below the dose which caused sedation in these animals.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA02 - Chlordiazepoxide
Route of Elimination
Chlordiazepoxide is excreted in the urine, with 1% to 2% unchanged and 3% to 6% as conjugate.
INCR DISAPPEARANCE RATES OF CHLORDIAZEPOXIDE & ITS METABOLITES WERE OBSERVED AFTER SINGLE PRETREATMENT DOSE TO MICE. INCR CLEARANCE WAS INSUFFICIENT TO EXPLAIN TOLERANCE OBSERVED AND THE POSSIBILITY OF ALTERED DRUG DISTRIBUTION BETWEEN BLOOD & BRAIN TISSUE CANNOT BE EXCLUDED.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 121
LEVELS OF (14)C WERE MAX IN TISSUES OF CYNOMOLGUS MONKEYS 2-6 HR AFTER ORAL DOSE OF CHLORDIAZEPOXIDE. BRAIN TO BLOOD CONCN RATIOS OF (14)C WERE GREATER THAN 1. CONCN OF (14)C WERE HIGHEST IN LIVER & KIDNEYS & LOWER IN HEART, LUNGS, SPLEEN, BRAIN, ADRENALS, PANCREAS & FAT. AFTER 24 HR, GI TRACT CONTAINED 15%, TISSUES 33%, URINE 34%, & FECES 1%.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 86
Chlordiazepoxide is absorbed more rapidly and predictably after oral than after intramuscular administration, but blood levels vary widely among individuals.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 216
PLASMA CONCENTRATION-TIME CURVES OF CHLORDIAZEPOXIDE WERE SATISFACTORILY DESCRIBED BY A BI-EXPONENTIAL EXPRESSION CONSISTENT WITH A TWO-COMPARTMENT MODEL SYSTEM AFTER INTRAVENOUS DOSES TO HEALTHY MALE VOLUNTEERS. MEAN HALF-LIFE VALUES OF THE DISTRIBUTION AND ELIMINATION PHASE WERE 0.25 AND 9.4 HOURS, RESPECTIVELY, WHILE MEAN VALUES FOR VOLUMES OF THE CENTRAL COMPARTMENT (V1) AND THE OVERALL DISTRIBUTION (VDBETA) WERE 18 AND 31% OF BODY WEIGHT. DRUG ABSORPTION FROM IM DOSES WAS COMPARATIVELY SLOW AND ADEQUATE DESCRIPTION OF RESULTING PLASMA LEVELS OF CHLORDIAZEPOXIDE REQUIRED THE INCORPORATION OF A TWO-COMPARTMENT "MUSCLE MODEL" WHICH INCLUDED PRECIPITATED AND SOLUBILIZED DRUG IN MUSCLE TISSUE. DISTRIBUTION VOLUMES OF CHLORDIAZEPOXIDE WERE SIGNIFICANTLY LARGER IN FEMALE SUBJECTS THAN IN THE MALES, SUGGESTING MORE EXTENSIVE DRUG DISTRIBUTION AMONG FEMALES. CHLORDIAZEPOXIDE IS TAKEN UP BY THE RED CELLS TO ONLY A LIMITED EXTENT.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 5: A Review of the Literature Published during 1976 and 1977. London: The Chemical Society, 1979., p. 17
For more Absorption, Distribution and Excretion (Complete) data for CHLORDIAZEPOXIDE (9 total), please visit the HSDB record page.
Hepatic.
IN MAN & DOGS, BIOTRANSFORMATION RESULTS IN SUCCESSIVE HYDROLYSIS OF METHYLAMINO-SUBSTITUENT IN 2-POSITION & HYDROLYTIC FISSION OF RESULTANT LACTAM; 7-CHLORO-1,3-DIHYDRO-5-PHENYL-2H-1,4-BENZODIAZEPINE-2-ONE-4-OXIDE & N-(2-AMINO-5-CHLORO-ALPHA-PHENYL-BENZYLIDENE) GLYCINE-N-OXIDE ARE EXCRETED IN URINE.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 415
IN DOGS, 1% OF A DOSE OF LIBRIUM FOUND IN URINE AS OXAZEPAM GLUCURONIDE AND A FURTHER 1% IN FECES AS FREE OXAZEPAM GLUCURONIDE. THESE MINOR METABOLITES PRESUMABLY ARISE VIA LACTAM BY STEPS INVOLVING REDUCTION OF N-OXIDE FUNCTION.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 415
YIELDS 2-AMINO-7-CHLORO-5-PHENYL-3H-1,4-BENZODIAZEPINE-4-OXIDE IN MAN, IN MONKEY. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-14
YIELDS N-(2-AMINO-5-CHLORO-ALPHA-PHENYLBENZYLIDENE)-GLYCINE-N-OXIDE IN PSEUDOMONAS, IN CLOSTRIDIUM. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. C-14
... THE MAJOR ACTIVE METABOLITE /OF CHLORDIAZEPOXIDE IS/ DESMETHYLCHLORDIAZEPOXIDE ...
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 216
24-48 hours
The elimination half-life of the major active metabolite, desmethylchlordiazepoxide, ranges from one to four days ...
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 216
PLASMA CONCENTRATION TIME CURVES OF CHLORDIAZEPOXIDE WERE SATISFACTORILY DESCRIBED BY A BI-EXPONENTIAL EXPRESSION CONSISTENT WITH A TWO COMPARTMENT MODEL SYSTEM AFTER INTRAVENOUS DOSES TO HEALTHY MALE VOLUNTEERS. MEAN HALF-LIFE VALUES OF THE DISTRIBUTION AND ELIMINATION PHASE WERE 0.25 AND 9.4 HOURS, RESPECTIVELY ...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 5: A Review of the Literature Published during 1976 and 1977. London: The Chemical Society, 1979., p. 17
THE DISPOSITION OF CHLORDIAZEPOXIDE (CDP), 50 MG INFUSED IV OVER 10 MINUTES, WAS STUDIED IN NORMAL SUBJECTS AND IN PATIENTS WITH BIOPSY-PROVEN CIRRHOSIS. IN THE NORMAL SUBJECTS, MEAN KINETIC PARAMETER WAS HALF-LIFE (BETA), 10.0 HOURS. VALUE IN CIRRHOTIC PATIENTS WAS HALF-LIFE (BETA), 34.9 HOURS.
PMID:455893 SELLERS EM ET AL; CLIN PHARMACOL THER 26 (2): 240-6 (1979)
Half-life: 5-30 hr /From table/
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 314
Chlordiazepoxide binds to stereospecific benzodiazepine (BZD) binding sites on GABA (A) receptor complexes at several sites within the central nervous system, including the limbic system and reticular formation. This results in an increased binding of the inhibitory neurotransmitter GABA to the GABA(A) receptor. BZDs, therefore, enhance GABA-mediated chloride influx through GABA receptor channels, causing membrane hyperpolarization. The net neuro-inhibitory effects result in the observed sedative, hypnotic, anxiolytic, and muscle relaxant properties.
IN METHYLATED SPINAL CORD FROM 9-15-DAY-OLD-RATS, CHLORDIAZEPOXIDE ENHANCED EVOKED SYNAPTIC RESPONSES MEDIATED BY GAMMA-AMINOBUTYRIC ACID (GABA) AND POTENTIATED GABA-INDUCED DEPOLARIZATION OF PRIMARY AFFERENTS AND HYPERPOLARIZATION OF MOTOR NEURONS. THE GABA-POTENTIATING EFFECT OF CHLORDIAZEPOXIDE WAS INCREASED BY ELEVATED CL- IN THE MEDIUM, BUT DID NOT DEPEND ON THE CONCENTRATION OF K+. APPARENTLY, CHLORDIAZEPOXIDE MAY PROLONG THE LIFE SPAN OF THE CHLORINE IONOPHORE COUPLED WITH GABA RECEPTORS OF THE SPINAL CORD.
ABRAMETS II, KOMISSAROV IV; BYULL EKSP BIOL MED 94 (10): 58-61 (1982)
Currently, there is general agreement that most, if not all of the actions of benzodiazepines are a result of potentiation of the neural inhibition that is mediated by gamma-aminobutyric acid (GABA). This view is supported by behavioral and electrophysiological evidence that the effects of benzodiazepines are reduced or prevented by prior treatment with antagonists of gamma-aminobutyric acid (eg, bicuculline) or inhibitors of the synthesis of the transmitter (eg, thiosemicarbazide). ... Most attention has been focused on the ability of benzodiazepines to potentiate the actions of gamma-aminobutyric acid on neurons at all levels of the neuraxis. ... A substantial body of biochemical evidence has accumulated that suggests a close molecular association between sites of action for gamma-aminobutyric acid and the benzodiazepines. /Benzodiazepines/
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 345
A large body of electrophysiological and biochemical observations has linked the actions of benzodiazepines to the functions of receptor chloride ionophore systems that are regulated by gamma-aminobutyric acid. The evidence includes the ability of various benzodiazepines to potentiate the effects of exogenous gamma-aminobutyric acid or to enhance gamma-aminobutyric acid mediated presynaptic and postsynaptic inhibitory pathways. Further, specific sites for benzodiazepines have been characterized in cell membranes from brain, and their properties can be modified by gamma-aminobutyric acid and by chloride or related ions that are known to carry current through channels that are regulated by gamma-aminobutyric acid. ... Two different types of receptors for gamma-aminobutyric acid /are detected/ in hippocampal pyramidal cells; diazepam potentiates somatic responses to gamma-aminobutyric acid that involve increases in chloride conductance ... potentiation of responses to gamma-aminobutyric acid requires concentrations of benzodiazepines 10 to 100 fold greater than those achieved in the CSF during therapy ... These observations suggest that the anticonvulsant effects of the benzodiazepines may not depend entirely upon actions on gamma-aminobutyric acid-ergic neurotransmission or on channels for chloride ions. /Benzodiazepines/
Gilman, A.G., L.S.Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 7th ed. New York: Macmillan Publishing Co., Inc., 1985., p. 465

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
14
PharmaCompass offers a list of Chlordiazepoxide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlordiazepoxide manufacturer or Chlordiazepoxide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlordiazepoxide manufacturer or Chlordiazepoxide supplier.
PharmaCompass also assists you with knowing the Chlordiazepoxide API Price utilized in the formulation of products. Chlordiazepoxide API Price is not always fixed or binding as the Chlordiazepoxide Price is obtained through a variety of data sources. The Chlordiazepoxide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chlordiazepoxide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chlordiazepoxide, including repackagers and relabelers. The FDA regulates Chlordiazepoxide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chlordiazepoxide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Chlordiazepoxide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Chlordiazepoxide supplier is an individual or a company that provides Chlordiazepoxide active pharmaceutical ingredient (API) or Chlordiazepoxide finished formulations upon request. The Chlordiazepoxide suppliers may include Chlordiazepoxide API manufacturers, exporters, distributors and traders.
click here to find a list of Chlordiazepoxide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Chlordiazepoxide DMF (Drug Master File) is a document detailing the whole manufacturing process of Chlordiazepoxide active pharmaceutical ingredient (API) in detail. Different forms of Chlordiazepoxide DMFs exist exist since differing nations have different regulations, such as Chlordiazepoxide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Chlordiazepoxide DMF submitted to regulatory agencies in the US is known as a USDMF. Chlordiazepoxide USDMF includes data on Chlordiazepoxide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Chlordiazepoxide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Chlordiazepoxide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Chlordiazepoxide Drug Master File in Japan (Chlordiazepoxide JDMF) empowers Chlordiazepoxide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Chlordiazepoxide JDMF during the approval evaluation for pharmaceutical products. At the time of Chlordiazepoxide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Chlordiazepoxide suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Chlordiazepoxide Drug Master File in Korea (Chlordiazepoxide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Chlordiazepoxide. The MFDS reviews the Chlordiazepoxide KDMF as part of the drug registration process and uses the information provided in the Chlordiazepoxide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Chlordiazepoxide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Chlordiazepoxide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Chlordiazepoxide suppliers with KDMF on PharmaCompass.
A Chlordiazepoxide CEP of the European Pharmacopoeia monograph is often referred to as a Chlordiazepoxide Certificate of Suitability (COS). The purpose of a Chlordiazepoxide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Chlordiazepoxide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Chlordiazepoxide to their clients by showing that a Chlordiazepoxide CEP has been issued for it. The manufacturer submits a Chlordiazepoxide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Chlordiazepoxide CEP holder for the record. Additionally, the data presented in the Chlordiazepoxide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Chlordiazepoxide DMF.
A Chlordiazepoxide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Chlordiazepoxide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Chlordiazepoxide suppliers with CEP (COS) on PharmaCompass.
A Chlordiazepoxide written confirmation (Chlordiazepoxide WC) is an official document issued by a regulatory agency to a Chlordiazepoxide manufacturer, verifying that the manufacturing facility of a Chlordiazepoxide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Chlordiazepoxide APIs or Chlordiazepoxide finished pharmaceutical products to another nation, regulatory agencies frequently require a Chlordiazepoxide WC (written confirmation) as part of the regulatory process.
click here to find a list of Chlordiazepoxide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Chlordiazepoxide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Chlordiazepoxide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Chlordiazepoxide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Chlordiazepoxide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Chlordiazepoxide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Chlordiazepoxide suppliers with NDC on PharmaCompass.
Chlordiazepoxide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chlordiazepoxide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chlordiazepoxide GMP manufacturer or Chlordiazepoxide GMP API supplier for your needs.
A Chlordiazepoxide CoA (Certificate of Analysis) is a formal document that attests to Chlordiazepoxide's compliance with Chlordiazepoxide specifications and serves as a tool for batch-level quality control.
Chlordiazepoxide CoA mostly includes findings from lab analyses of a specific batch. For each Chlordiazepoxide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chlordiazepoxide may be tested according to a variety of international standards, such as European Pharmacopoeia (Chlordiazepoxide EP), Chlordiazepoxide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chlordiazepoxide USP).

