
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
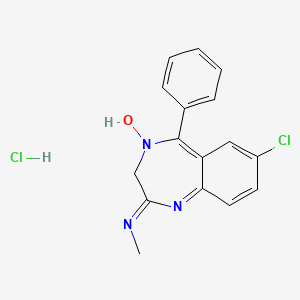

1. 7 Chloro 2 Methylamino 5 Phenyl 3h 1,4 Benzodiazepine 4 Oxide
2. 7 Chloro N Methyl 5 Phenyl 3h 1,4 Benzodiazepin 2 Amine 4 Oxide
3. 7-chloro-2-methylamino-5-phenyl-3h-1,4-benzodiazepine-4-oxide
4. 7-chloro-n-methyl-5-phenyl-3h-1,4-benzodiazepin-2-amine 4-oxide
5. Chlordiazepoxide
6. Chlordiazepoxide Hydrobromide
7. Chlordiazepoxide Monohydrochloride
8. Chlordiazepoxide Perchlorate
9. Chlozepid
10. Elenium
11. Hydrobromide, Chlordiazepoxide
12. Hydrochloride, Chlordiazepoxide
13. Librium
14. Methaminodiazepoxide
15. Monohydrochloride, Chlordiazepoxide
16. Perchlorate, Chlordiazepoxide
1. 438-41-5
2. Clopoxide Chloride
3. Librium
4. Chlordiazachel
5. Calmoden
6. Chlordiazepoxide Hcl
7. Protensin
8. Contol
9. Retcol
10. Chlordiazepoksid
11. A-poxide
12. Droxol Hydrochloride
13. Lygen
14. Napoton Hydrochloride
15. Libritabs Hydrochloride
16. Risachief Hydrochloride
17. Sk-lygen
18. Ro 5-0690
19. Chlorodiazepoxide Hydrochloride
20. Chlordiazepoxide Monohydrochloride
21. Methaminodiazepoxide Hydrochloride
22. Mfm6k1xwdk
23. Nsc-115748
24. 7-chloro-2-(methylamino)-5-phenyl-3h-1,4-benzodiazepine 4-oxide Monohydrochloride
25. Radepur
26. 3h-1,4-benzodiazepin-2-amine, 7-chloro-n-methyl-5-phenyl-, 4-oxide, Monohydrochloride
27. Ro-5-0690
28. Benzodiapin
29. Ansiacal
30. Equibral
31. Labican
32. Lentotran
33. Novosed
34. Psichial
35. Reliberan
36. Sophiamin
37. Tensinyl
38. Timosin
39. Trakipeal
40. Viansin
41. Cebrum
42. Murcil
43. Seren Vita
44. J-liberty
45. Diazachel (obs.)
46. Librium Hydrochloride
47. Unii-mfm6k1xwdk
48. Einecs 207-117-8
49. Nsc 115748
50. 7-chloro-4-hydroxy-n-methyl-5-phenyl-3h-1,4-benzodiazepin-2-imine;hydrochloride
51. Component Of Librax
52. Librium (tn)
53. Librium, Hydrochloride
54. Schembl8935
55. Chlordiazepoxide Hydrochloride [usan:usp:ban:jan]
56. Chloridiazepoxide Hydrochloride
57. Methaminodiazepine Hydrochloride
58. Chebi:3612
59. Chembl1200703
60. Nsc115748
61. Akos015895152
62. 3h-1,4-benzodiazepine, 7-chloro-2-methylamino-5-phenyl-, 4-oxide, Monohydrochloride
63. Chlordiazepoxide Hydrochloride Civ
64. Chlordiazepoxide Hydrochloride (jan/usp)
65. Chlordiazepoxide Hydrochloride [mi]
66. Chlordiazepoxide Hydrochloride [jan]
67. Db-051172
68. Chlordiazepoxide Hydrochloride [usan]
69. Chlordiazepoxide Hydrochloride [vandf]
70. Chlordiazepoxide Hydrochloride [mart.]
71. C-2992
72. Chlordiazepoxide Hydrochloride [usp-rs]
73. Chlordiazepoxide Hydrochloride [who-dd]
74. D00693
75. Wln: T67 Gn Jn Ihj Cg Hm1 Jo Kr &gh
76. Chlordiazepoxide Hydrochloride Civ [usp-rs]
77. Chlordiazepoxide Hydrochloride [ep Monograph]
78. Chlordiazepoxide Hydrochloride [orange Book]
79. Chlordiazepoxide Hydrochloride [usp Monograph]
80. Q27106148
81. 3h-1, 7-chloro-n-methyl-5-phenyl-, 4-oxide, Monohydrochloride
82. 7-chloro-2-(methylamino)-5-phenyl-3h-1, 4-oxide, Hydrochloride
83. Chlordiazepoxide Hydrochloride, Drug Standard, 1.0 Mg/ml In Methanol
84. 3h-1, 7-chloro-2-(methylamino)-5-phenyl-, 4-oxide, Monohydrochloride
85. (1e,4e)-7-chloro-2-(methylamino)-5-phenyl-3h-benzo[e][1,4]diazepine 4-oxide Hydrochloride
86. 7-chloro-2-(methylamino)-5-phenyl-3h-1,4-benzodiazepine-4-oxide Hydrochloride
87. 7-chloro-2-(methylamino)-5-phenyl-3h-benzo[e][1,4]diazepine 4-oxide Hydrochloride
88. 7-chloro-n-methyl-4-oxido-5-phenyl-3h-1,4-benzodiazepin-4-ium-2-amine;hydrochloride
89. Chlordiazepoxide Hydrochloride, European Pharmacopoeia (ep) Reference Standard
90. Chlordiazepoxide Hydrochloride, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 336.2 g/mol |
|---|---|
| Molecular Formula | C16H15Cl2N3O |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 335.0592175 g/mol |
| Monoisotopic Mass | 335.0592175 g/mol |
| Topological Polar Surface Area | 48.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 580 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Chlordiazepoxide hydrochloride |
| PubMed Health | Chlordiazepoxide (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Chlordiazepoxide hydrochloride is the prototype for the benzodiazepine compounds. It is a versatile therapeutic agent of proven value for the relief of anxiety. Chlordiazepoxide hydrochloride is among the safer of the effective psychopharmacologic co... |
| Active Ingredient | Chlordiazepoxide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 5mg; 25mg; 10mg |
| Market Status | Prescription |
| Company | Usl Pharma; Barr |
| 2 of 4 | |
|---|---|
| Drug Name | Librium |
| Drug Label | Librium, the original chlordiazepoxide HCl and prototype for the benzodiazepine compounds, was synthesized and developed at Hoffmann-La Roche Inc. It is a versatile therapeutic agent of proven value for the relief of anxiety. Librium is among the... |
| Active Ingredient | Chlordiazepoxide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
| 3 of 4 | |
|---|---|
| Drug Name | Chlordiazepoxide hydrochloride |
| PubMed Health | Chlordiazepoxide (By mouth) |
| Drug Classes | Antianxiety |
| Drug Label | Chlordiazepoxide hydrochloride is the prototype for the benzodiazepine compounds. It is a versatile therapeutic agent of proven value for the relief of anxiety. Chlordiazepoxide hydrochloride is among the safer of the effective psychopharmacologic co... |
| Active Ingredient | Chlordiazepoxide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 5mg; 25mg; 10mg |
| Market Status | Prescription |
| Company | Usl Pharma; Barr |
| 4 of 4 | |
|---|---|
| Drug Name | Librium |
| Drug Label | Librium, the original chlordiazepoxide HCl and prototype for the benzodiazepine compounds, was synthesized and developed at Hoffmann-La Roche Inc. It is a versatile therapeutic agent of proven value for the relief of anxiety. Librium is among the... |
| Active Ingredient | Chlordiazepoxide hydrochloride |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 25mg; 5mg; 10mg |
| Market Status | Prescription |
| Company | Valeant Pharm Intl |
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
14
PharmaCompass offers a list of Chlordiazepoxide HCl API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlordiazepoxide HCl manufacturer or Chlordiazepoxide HCl supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlordiazepoxide HCl manufacturer or Chlordiazepoxide HCl supplier.
PharmaCompass also assists you with knowing the Chlordiazepoxide HCl API Price utilized in the formulation of products. Chlordiazepoxide HCl API Price is not always fixed or binding as the Chlordiazepoxide HCl Price is obtained through a variety of data sources. The Chlordiazepoxide HCl Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chlordiazepoxide HCl manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chlordiazepoxide HCl, including repackagers and relabelers. The FDA regulates Chlordiazepoxide HCl manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chlordiazepoxide HCl API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Chlordiazepoxide HCl manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Chlordiazepoxide HCl supplier is an individual or a company that provides Chlordiazepoxide HCl active pharmaceutical ingredient (API) or Chlordiazepoxide HCl finished formulations upon request. The Chlordiazepoxide HCl suppliers may include Chlordiazepoxide HCl API manufacturers, exporters, distributors and traders.
click here to find a list of Chlordiazepoxide HCl suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Chlordiazepoxide HCl DMF (Drug Master File) is a document detailing the whole manufacturing process of Chlordiazepoxide HCl active pharmaceutical ingredient (API) in detail. Different forms of Chlordiazepoxide HCl DMFs exist exist since differing nations have different regulations, such as Chlordiazepoxide HCl USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Chlordiazepoxide HCl DMF submitted to regulatory agencies in the US is known as a USDMF. Chlordiazepoxide HCl USDMF includes data on Chlordiazepoxide HCl's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Chlordiazepoxide HCl USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Chlordiazepoxide HCl suppliers with USDMF on PharmaCompass.
A Chlordiazepoxide HCl CEP of the European Pharmacopoeia monograph is often referred to as a Chlordiazepoxide HCl Certificate of Suitability (COS). The purpose of a Chlordiazepoxide HCl CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Chlordiazepoxide HCl EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Chlordiazepoxide HCl to their clients by showing that a Chlordiazepoxide HCl CEP has been issued for it. The manufacturer submits a Chlordiazepoxide HCl CEP (COS) as part of the market authorization procedure, and it takes on the role of a Chlordiazepoxide HCl CEP holder for the record. Additionally, the data presented in the Chlordiazepoxide HCl CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Chlordiazepoxide HCl DMF.
A Chlordiazepoxide HCl CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Chlordiazepoxide HCl CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Chlordiazepoxide HCl suppliers with CEP (COS) on PharmaCompass.
Chlordiazepoxide HCl Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chlordiazepoxide HCl GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chlordiazepoxide HCl GMP manufacturer or Chlordiazepoxide HCl GMP API supplier for your needs.
A Chlordiazepoxide HCl CoA (Certificate of Analysis) is a formal document that attests to Chlordiazepoxide HCl's compliance with Chlordiazepoxide HCl specifications and serves as a tool for batch-level quality control.
Chlordiazepoxide HCl CoA mostly includes findings from lab analyses of a specific batch. For each Chlordiazepoxide HCl CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chlordiazepoxide HCl may be tested according to a variety of international standards, such as European Pharmacopoeia (Chlordiazepoxide HCl EP), Chlordiazepoxide HCl JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chlordiazepoxide HCl USP).

