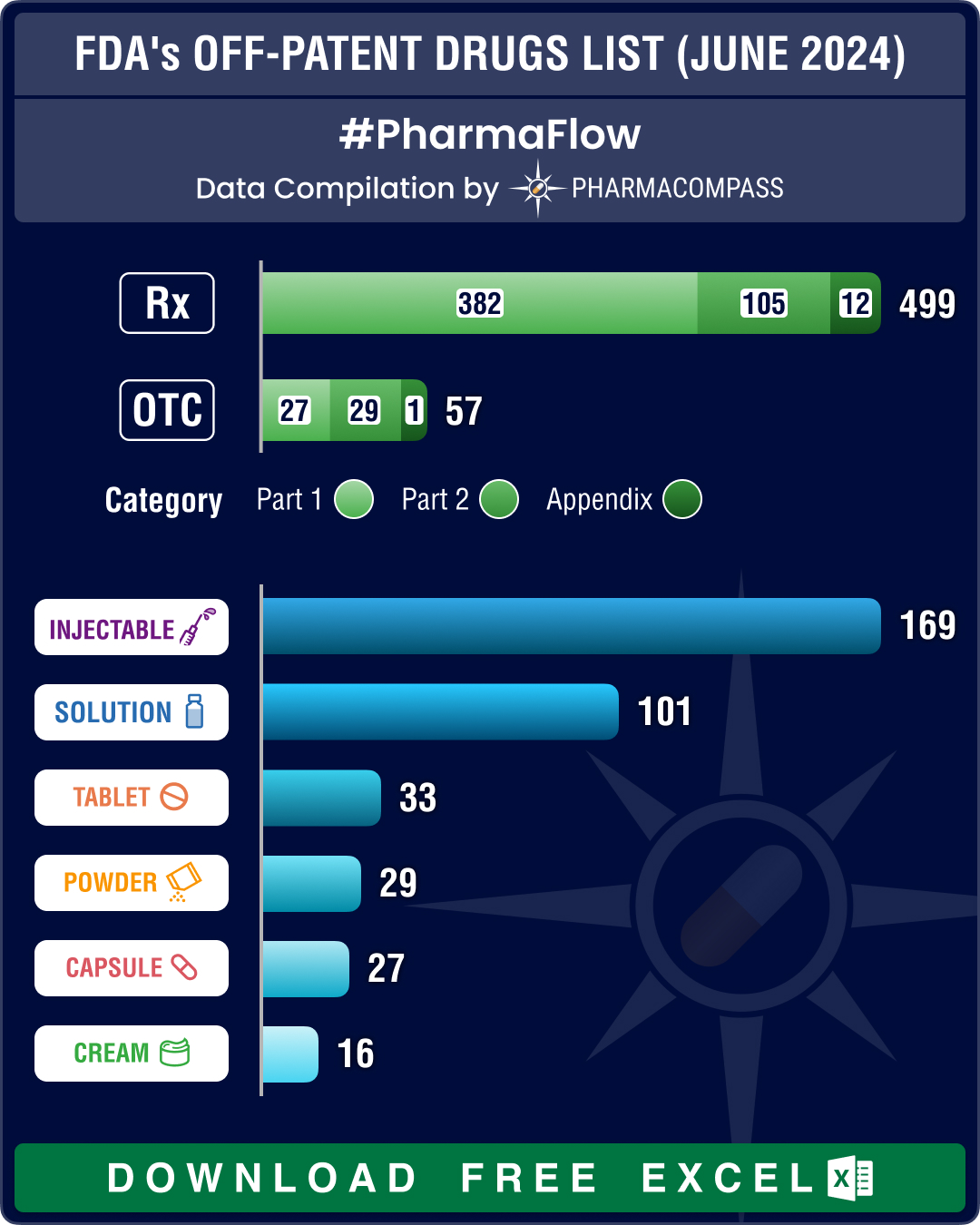
FDA’s December 2025 OPOE list features 784 prescription drugs, 73 OTC drugs
This
week, PharmaCompass brings you key highlights of the US Food and Drug Administration’s D
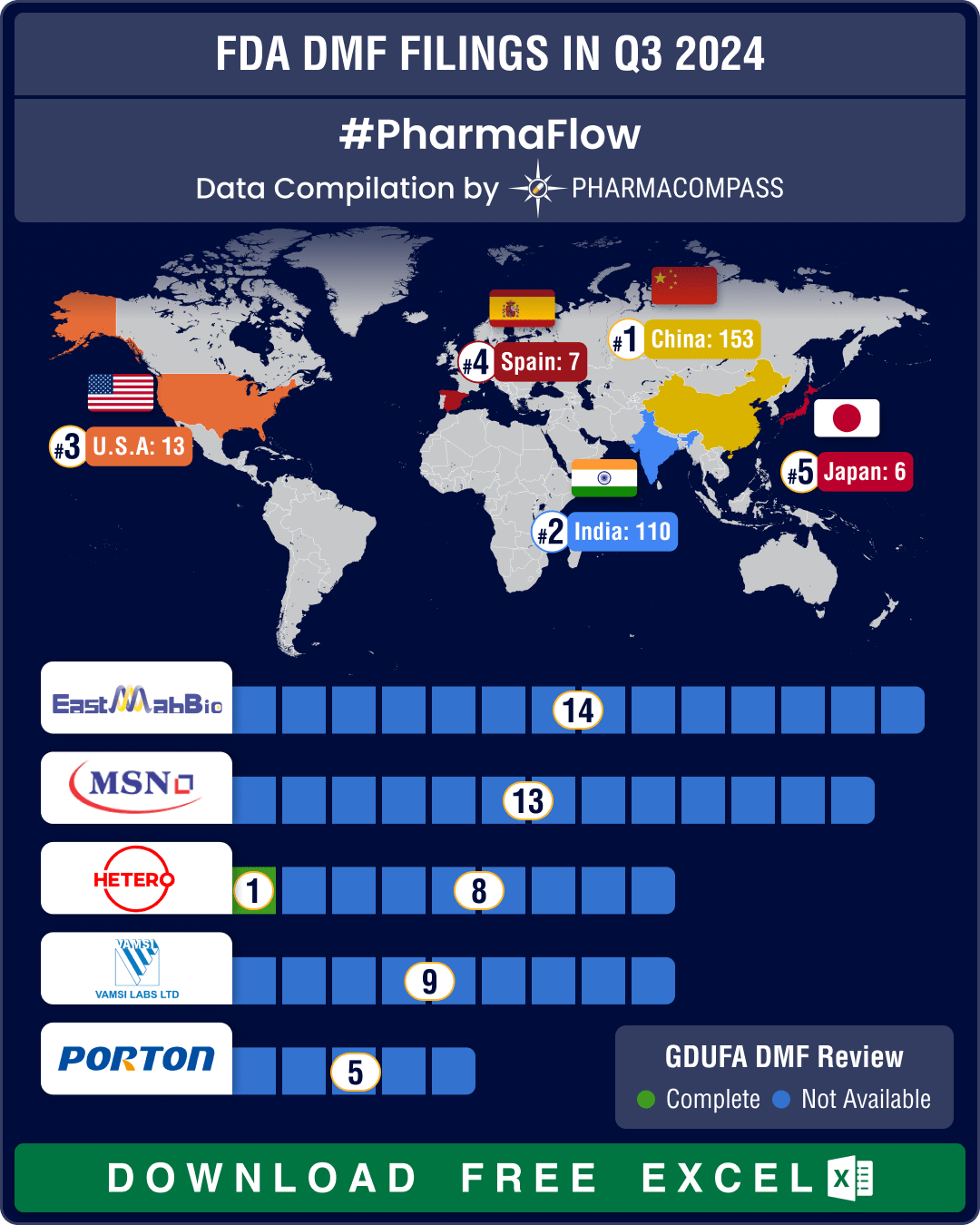
DMF filings hit all-time high in Q3 2024; China tops list with 58% increase in Type II submissions
Drug Master Files, or DMFs, are confidential documents that play a crucial role in the pharmaceutica


 Market Place
Market Place Sourcing Support
Sourcing Support