By PharmaCompass
2020-12-31
Impressions: 8054
The year 2020 was an eventful year for the pharmaceutical industry, with several companies across the world working at a feverish pace to find a treatment or a vaccine for the raging Covid-19, which has so far taken over 1.79 million lives worldwide.
With countries imposing lockdowns and regulators putting on-site inspections on hold, we were expecting far lower new drug approvals in mid-2020. But our mid-2020 recap published in July, which looked at new drug approvals by the US Food and Drug Administration (FDA) and European Medical Agency (EMA), found that the FDA had approved 33 new drugs by the end of June. This put the approvals within the ballpark of the past two years.
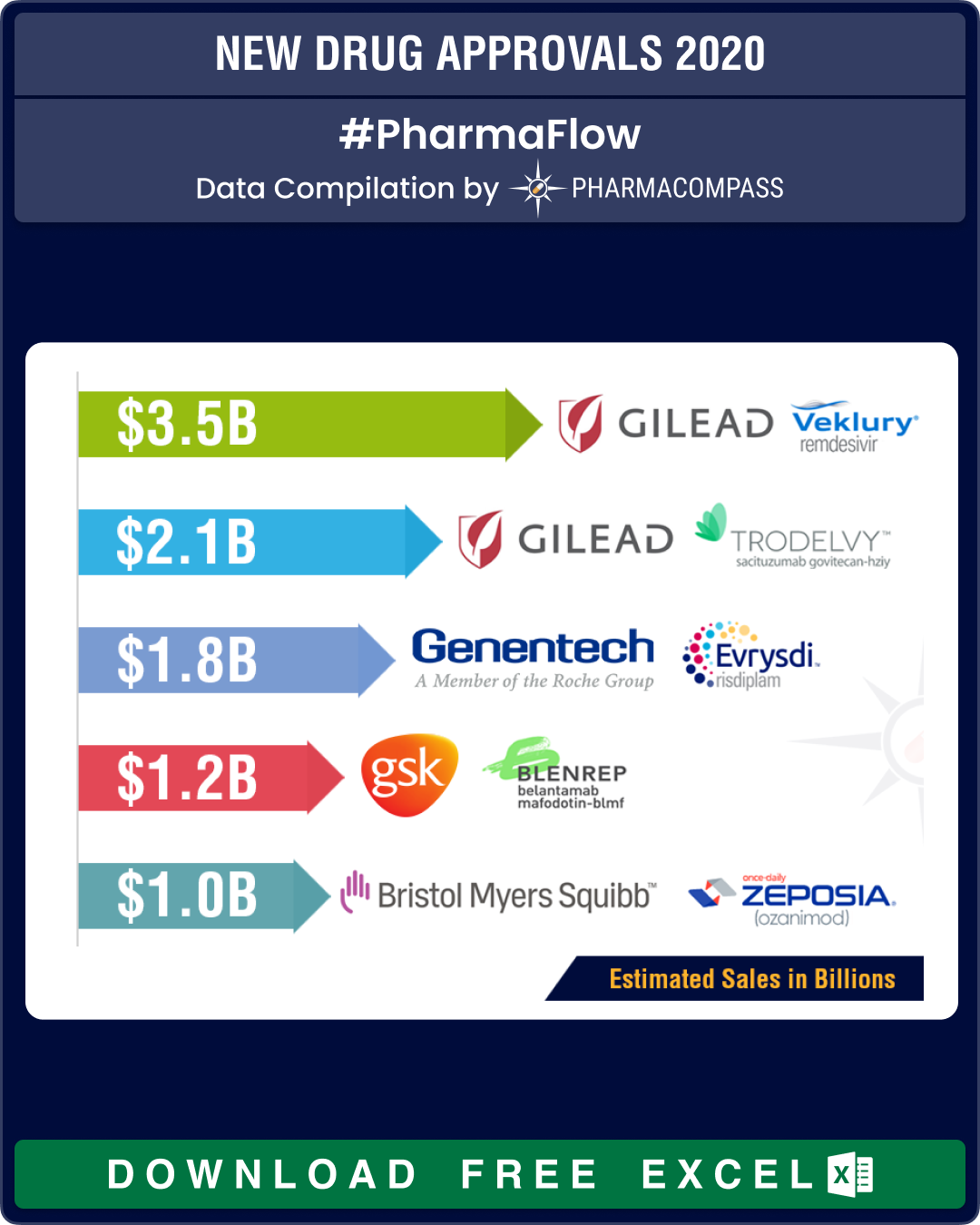
This week, we bring you a roundup of 2020, a tumultuous year when 58 drugs (53 approvals by the Center for Drug Evaluation and Research and 5 by the Center for Biologics Evaluation and Research) bagged FDA’s new drug approvals. While this number is lower than the number of drugs approved in 2018 (62), it is higher than the number for 2019 (54). Out of this, while 23 approvals were in the field of oncology, 9 were for infectious diseases and infections, 8 for genetic diseases, 7 for neurology, 3 for immunology and 2 for gastroenterology.
View New Drug Approvals in 2020 with Estimated Sales (Free Excel Available)
A year marked by EUA
With the pandemic raging across the world, emergency use authorizations (EUAs) dominated news headlines in 2020 — the FDA issued 10 EUAs, with the most prominent being those issued to Pfizer-BioNTech and Moderna for their Covid-19 vaccines.
EMA was busy as well since they issued 75 positive opinions
with Novartis leading
the pack with 8, followed by Pfizer and Sanofi which received 4 each.
The EUAs came with their own set of controversies. In March, the FDA had issued an EUA “for oral formulations of chloroquine phosphate and hydroxychloroquine sulfate for the treatment of” Covid-19. However, by June, FDA had revoked the EUA, as the agency determined that chloroquine and hydroxychloroquine were not likely to be effective in treating Covid-19 for the authorized uses in the EUA.
Amongst treatments for Covid-19, in May the FDA authorized the emergency use of Gilead’s antiviral drug remdesivir. In our mid-2020 recap, Gilead’s remdesivir was on top of our list of top-selling drugs after it received an EUA from the FDA.
In October, remdesivir became the first drug to be approved by the FDA for treatment of Covid-19 patients requiring hospitalization. While analysts predicted US$ 3.5 billion in revenue in early October, the future of this drug as a treatment for Covid-19 in hospitalized patients remains uncertain, especially in wake of results from the World Health Organization (WHO)-led Solidarity Trial that said Gilead’s remdesivir had little or no effect on the 28-day mortality or length of hospital stays for Covid-19 patients. The FDA approved remdesivir for hospitalized patients a week after the WHO results.
View New Drug Approvals in 2020 with Estimated Sales (Free Excel Available)
Gilead US$ 21 billion Immunomedics acquisition
Immunomedics' antibody-drug conjugate (ADC) — Trodelvy (sacituzumab govitecan-hziy) — was approved by the FDA in April this year for the treatment of adult patients with metastatic triple-negative breast cancer who have received at least two prior therapies for the disease. Such tumor types account for 15 to 20 percent of breast cancers. Trodelvy follows remdesivir in our list of FDA approved drugs in 2020 with the highest sales potential. The current forecast for Trodelvy sales is US$ 2.151 billion by 2026.
In September, Gilead made a big move and acquired biotech company Immunomedics Inc for US$ 21 billion. The transaction, which was completed in October, will strengthen Gilead’s cancer portfolio and add another potential blockbuster to it.
Immunomedics plans to submit a supplemental Biologics License Application (BLA) to support full approval of Trodelvy in the US over the next quarter. According to a statement, Immunomedics is also on track to file for regulatory approval of the drug in Europe in the first half of 2021. Moreover, ongoing studies are also evaluating the potential of Trodelvy as a treatment for non-small cell lung cancer and other types of solid tumors.
View New Drug Approvals in 2020 with Estimated Sales (Free Excel Available)
Roche-PTC Therapeutics’ risdiplam bags approval
Following Troveldy in sales potential for drugs approved by the FDA is Roche and PTC Therapeutics’ drug Evrysdi (risdiplam), the first oral medicine approved for the rare genetic disease, spinal muscular atrophy, which until four years ago had no available treatments.
The approval of Evrysdi presents patients and their families with a unique choice between a one-time gene therapy, an RNA-based drug infused three times a year at the doctor’s office and a daily medicine taken at home.
Roche priced the drug by patient weight, with a maximum cost of US$ 340,000 per year — substantially lesser than the competing (and approved) therapies from Biogen and Novartis.
View New Drug Approvals in 2020 with Estimated Sales (Free Excel Available)
Vertex’s Kaftrio bags EMA approval
Earlier this year, PharmaCompass had published its compilation of sales forecasts for the new drugs approved by the FDA in 2019. The list was led by Vertex’s cystic fibrosis treatment — Trikafta — which was expected to have sales of US$ 3.935 billion by 2024.
Trikafta is a combination of ivacaftor, tezacaftor and elexacaftor and its stellar clinical data made the FDA approve the drug within three months of Vertex’s application filing and five months before FDA’s action date.
In June 2020, EMA’s CHMP adopted a positive opinion, recommending the granting of a marketing authorization for Vertex’s combination, which will be marketed as Kaftrio.
View New Drug Approvals in 2020 with Estimated Sales (Free Excel Available)
A year of multiple setbacks
Not everything went smoothly in 2020. In fact, the year saw several setbacks — almost 44 drugs were not granted approval by the FDA. Bristol Myers Squibb was one such company that received setbacks. As part of Bristol’s US$ 74 billion acquisition of Celgene, the New York-headquartered drug company offered Celgene shareholders Contingent Value Rights or CVRs. But to realize the US$ 9-a piece payment, approvals for three ex-Celgene drugs must meet their pre-specified deadlines.
While in March, the FDA approved Bristol’s ozanimod, a sphingosine-1-phosphate receptor agonist for the treatment of relapsing multiple sclerosis, well ahead of the December 31, 2020 deadline, in May, Bristol Myers Squibb and bluebird bio, Inc announced that they have received a Refusal to File letter from FDA regarding the Biologics License Application (BLA) for their CAR-T therapy — idecabtagene vicleucel (ide-cel) — for patients with heavily pre-treated relapsed and refractory multiple myeloma, which was submitted in March 2020. Upon preliminary review, the FDA determined that the Chemistry, Manufacturing and Control (CMC) module of the BLA requires further details to complete the review.
This was followed by Bristol Myers announcing that the FDA has extended the action date by three months for the BLA for lisocabtagene maraleucel (liso-cel), a CD19-directed CAR-T therapy for the treatment of adults with relapsed or refractory (R/R) large B-cell lymphoma after at least two prior therapies. The FDA had then set the new Prescription Drug User Fee Act (PDUFA) action date as November 16, 2020. However, on that day, FDA informed the company that its review of the BLA for liso-cel will not be completed by November 16.
The FDA approval of liso-cel by December 31, 2020 is one of the required remaining milestones of the CVRs issued upon the close of the Celgene acquisition in the fourth quarter of 2019. The other is FDA approval of ide-cel by March 31, 2021.
FDA declines approval to Novartis’ inclisiran: Recently, there was news that the FDA declined to approve Swiss drugmaker Novartis AG’s lipid-lowering therapy, inclisiran (branded as Leqvio). The drug came to Novartis' fold through the US$ 9.7 billion acquisition of The Medicines Company last year. The drug has been cleared by the European Commission.
In a statement, Novartis said the FDA has not raised any concerns related to the efficacy or safety of inclisiran. The complete response letter (CRL) is due to unresolved facility inspection-related conditions. No onsite inspection was conducted by the FDA, the company said.
View New Drug Approvals in 2020 with Estimated Sales (Free Excel Available)
Our view
Like the drug companies, regulators across the world also worked at a frenetic pace in order to accelerate EUAs and drug approvals, especially for treatments and vaccines for Covid-19.
Apart from Covid-19, the year saw wider adoption and approvals for cell and gene therapies along with approvals of several innovative medicines like relugolix (the first oral gonadotropin-releasing hormone receptor antagonist for the treatment of adult patients with advanced prostate cancer), berotralstat the first oral once daily plasma kallikrein inhibitor to prevent attacks of hereditary angioedema in adults and pediatric patients 12 years and older, lumasiran (an HAO1-directed small interfering ribonucleic acid indicated for the treatment of primary hyperoxaluria type 1 to lower urinary oxalate levels in pediatric and adult patients) and osilodrostat for the treatment of adults with Cushing’s disease).
Moreover, there were several interesting ‘non-Covid’ medical breakthroughs that took place during 2020. One such breakthrough is a single pill that combines four medications meant to lower blood pressure and cholesterol and aspirin that was found to cut the risk of heart disease.
While the agility shown by pharma companies and regulators was undoubtedly quite impressive, with many countries granting EUA to vaccines for Covid-19, the ongoing pandemic did put mankind in a bind for several months. And if the words of the WHO director general Tedros Adhanom Ghebreyesus are to be believed, the coronavirus crisis will not be the last pandemic and attempts to improve human health are “doomed” without tackling climate change and animal welfare.
If that really proves to be the case, the pharma industry has a lot to learn from this pandemic, and science has a lot to demonstrate if mankind is to emerge unscathed from such adversities.
View New Drug Approvals in 2020 with Estimated Sales (Free Excel Available)
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : New Drugs Approvals 2020 by PharmaCompass is licensed under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







