
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
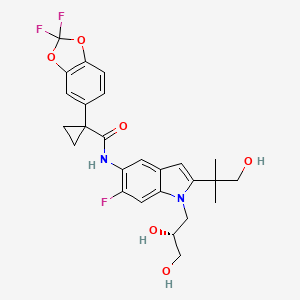

1. Vx-661
1. Vx-661
2. 1152311-62-0
3. Tezacaftor (vx-661)
4. Tezacaftor [usan]
5. (r)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl)cyclopropanecarboxamide
6. Vx661
7. Vx 661
8. 8rw88y506k
9. 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-n-{1-[(2r)-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)indol-5-yl}cyclopropane-1-carboxamide
10. Unii-8rw88y506k
11. 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-n-[1-[(2r)-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)indol-5-yl]cyclopropane-1-carboxamide
12. Cv6
13. Cyclopropanecarboxamide, 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-n-(1-((2r)-2,3-dihydroxypropyl)-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1h-indol-5-yl)-
14. Tezacaftor [mi]
15. Tezacaftor [inn]
16. Tezacaftor (usan/inn)
17. Tezacaftor [who-dd]
18. Schembl322362
19. Vx 661;vx661;tezacaftor
20. Chembl3544914
21. Schembl10034144
22. Tezacaftor [orange Book]
23. Gtpl10199
24. Dtxsid40673070
25. Bdbm281054
26. Symkevi Component Tezacaftor
27. Bcp07173
28. Cyclopropanecarboxamide, 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-n-[1-[(2r)-2,3-dihydroxypropyl]-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1h-indol-5-yl]-
29. Ex-a1759
30. Trikafta Component Tezacaftor
31. Mfcd23106064
32. S7059
33. Us10022352, Compound 315
34. Zinc68206930
35. Ccg-269854
36. Cs-1078
37. Db11712
38. Tezacaftor Component Of Trikafta
39. 1-(2,2-difluoro-2h-1,3-benzodioxol-5-yl)-n-{1-[(2r)-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl}cyclopropane-1-carboxamide
40. Ac-32773
41. As-35211
42. Hy-15448
43. Example 315 [us20090131492a1]
44. Sw219937-1
45. D11041
46. A857959
47. Q27270940
48. (r)-1 (2,2-difluorobenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl)cyclopropanecarboxamide
49. (r)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl)cyclopropane-1-carboxamide
50. 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-~{n}-[1-[(2~{r})-2,3-dihydroxypropyl]-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)indol-5-yl]cyclopropane-1-carboxamide
51. 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-n-(1-((2r)-2,3-dihydroxypropyl)-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1h-indol-5-yl)cyclopropanecarboxamide
52. 1-(2,2-difluoro-1,3-benzodioxol-5-yl)-n-[1-[(2r)-2,3-dihydroxypropyl]-6-fluoro-2-(2-hydroxy-1,1-dimethylethyl)-1h-indol-5-yl]-cyclopropanecarboxamide
53. Vx661; Vx 661; Tezacaftor;(r)-1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)-n-(1-(2,3-dihydroxypropyl)-6-fluoro-2-(1-hydroxy-2-methylpropan-2-yl)-1h-indol-5-yl)cyclopropanecarboxamide
| Molecular Weight | 520.5 g/mol |
|---|---|
| Molecular Formula | C26H27F3N2O6 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 520.18212107 g/mol |
| Monoisotopic Mass | 520.18212107 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 858 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tezacaftor is combined with ivacaftor in one product for the treatment of cystic fibrosis (CF) in patients aged 12 years or older with two copies of the _F508del_ gene mutation or at least one mutation in the CFTR gene that is responsive to this drug. Tezacaftor, when used in combination with ivacaftor and [elexacaftor] in the product Trikafta, is also indicated for the treatment of CF in patients 12 years of age and older that have at least one _F508del_ mutation in the CFTR gene.
FDA Label
Clinical studies have shown a significant decrease in sweat chloride and an increase in the forced expiratory volume (FEV), a measure of lung function, following Tevacaftor/Ivacaftor therapy. Phase 3 clinical studies have shown that a significant increase in forced expiratory volume was attained at 4 and 8 weeks after initiating this drug. The above effects lead to improvement of the respiratory symptoms of cystic fibrosis. Tezacaftor does not induce clinically significant QT prolongation. When given with ivacaftor, tezacaftor can lead to liver transaminase elevations. Testing of transaminases (ALT and AST) levels should occur before starting this combination every 3 months during the first year of treatment, and every year afterwards. Patients with a history of transaminase elevations should be monitored more frequently.
Absorption
The Cmax, Tmax and AUC of tezacaftor, when administered with ivacaftor, are 5.95 mcg/ml, 2-6 h, and 84.5 mcg.h/ml respectively. Exposure of tezacaftor/ivacaftor increases 3-fold when it is administered with a high-fat meal.
Route of Elimination
After oral administration, the majority of tezacaftor dose (72%) is found excreted in the feces either unchanged or as its metabolite, M2. About 14% of the administered dose is found excreted in the urine as the metabolite, M2. It was noted that less than 1% of the administered dose is excreted unchanged in the urine and thus, renal excretion is not the major elimination pathway.
Volume of Distribution
The apparent volume of distribution of tezacaftor was 271 L in a study of patients in the fed state who received 100 mg of tezacaftor every 12 hours.
Clearance
The apparent clearance of tezacaftor has been measured at 1.31 L/h for patients in the fed state during a clinical trial.
Tezacaftor is metabolized extensively in humans by the action of CYP3A4 and CYP3A5. There are three main circulating metabolites; M1, M2, and M5. The M1 is an active metabolite with similar activity to the parent drug, tezacaftor. The M2 metabolite is significantly less active and M5 is considered an inactive metabolite. An additional circulating metabolite, M3, corresponding to the glucuronide form of tezacaftor.
The apparent half-life of tezacaftor is approximately 57.2 hours.
The transport of charged ions across cell membranes is normally achieved through the actions of the cystic fibrosis transmembrane regulator (CFTR) protein. This protein acts as a channel and allows for the passage of chloride and sodium. This process affects the movement of water in and out of the tissues and impacts the production of mucus that lubricates and protects certain organs and body tissues, including the lungs. In the _F508del_ mutation of the CFTR gene, one amino acid is deleted at the position 508, therefore, the CFTR channel function is compromised, resulting in thickened mucus secretions. CFTR correctors such as tezacaftor aim to repair F508del cellular misprocessing. This is done by modulating the position of the CFTR protein on the cell surface to the correct position, allowing for adequate ion channel formation and increased in water and salt movement through the cell membrane. The concomitant use of ivacaftor is intended to maintain an open channel, increasing the transport of chloride, reducing thick mucus production.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
31
PharmaCompass offers a list of Tezacaftor API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tezacaftor manufacturer or Tezacaftor supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tezacaftor manufacturer or Tezacaftor supplier.
PharmaCompass also assists you with knowing the Tezacaftor API Price utilized in the formulation of products. Tezacaftor API Price is not always fixed or binding as the Tezacaftor Price is obtained through a variety of data sources. The Tezacaftor Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tezacaftor manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tezacaftor, including repackagers and relabelers. The FDA regulates Tezacaftor manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tezacaftor API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tezacaftor manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tezacaftor supplier is an individual or a company that provides Tezacaftor active pharmaceutical ingredient (API) or Tezacaftor finished formulations upon request. The Tezacaftor suppliers may include Tezacaftor API manufacturers, exporters, distributors and traders.
click here to find a list of Tezacaftor suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tezacaftor DMF (Drug Master File) is a document detailing the whole manufacturing process of Tezacaftor active pharmaceutical ingredient (API) in detail. Different forms of Tezacaftor DMFs exist exist since differing nations have different regulations, such as Tezacaftor USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tezacaftor DMF submitted to regulatory agencies in the US is known as a USDMF. Tezacaftor USDMF includes data on Tezacaftor's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tezacaftor USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tezacaftor suppliers with USDMF on PharmaCompass.
A Tezacaftor written confirmation (Tezacaftor WC) is an official document issued by a regulatory agency to a Tezacaftor manufacturer, verifying that the manufacturing facility of a Tezacaftor active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Tezacaftor APIs or Tezacaftor finished pharmaceutical products to another nation, regulatory agencies frequently require a Tezacaftor WC (written confirmation) as part of the regulatory process.
click here to find a list of Tezacaftor suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tezacaftor as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tezacaftor API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tezacaftor as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tezacaftor and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tezacaftor NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tezacaftor suppliers with NDC on PharmaCompass.
Tezacaftor Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tezacaftor GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tezacaftor GMP manufacturer or Tezacaftor GMP API supplier for your needs.
A Tezacaftor CoA (Certificate of Analysis) is a formal document that attests to Tezacaftor's compliance with Tezacaftor specifications and serves as a tool for batch-level quality control.
Tezacaftor CoA mostly includes findings from lab analyses of a specific batch. For each Tezacaftor CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tezacaftor may be tested according to a variety of international standards, such as European Pharmacopoeia (Tezacaftor EP), Tezacaftor JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tezacaftor USP).

