
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada
0
South Africa
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
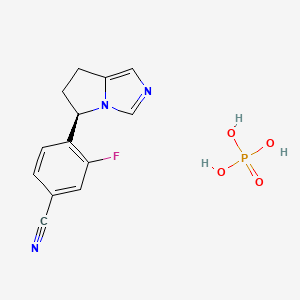

1. (+)-osilodrostat
2. 4-((5r)-6,7-dihydro-5h-pyrrolo(1,2-c)imidazol-5-yl)-3-fluoro-benzonitrile
3. Benzonitrile, 4-((5r)-6,7-dihydro-5h-pyrrolo(1,2-c)imidazol-5-yl)-3-fluoro-
4. Isturisa
5. Lci699
6. Osilodrostat
1. 1315449-72-9
2. Lci699-aza
3. Unii-y6581yaw9v
4. Osilodrostat Phosphate [usan]
5. Y6581yaw9v
6. Isturisa
7. Osilodrostat(lci699) Phosphate
8. Osilodrostat (phosphate)
9. 4-((5r)-6,7-dihydro-5h-pyrrolo(1,2-c)imidazol-5-yl)-3-fluorobenzonitrile Dihydrogen Phosphate
10. 4-[(5r)-6,7-dihydro-5h-pyrrolo[1,2-c]imidazol-5-yl]-3-fluorobenzonitrile;phosphoric Acid
11. Benzonitrile, 4-((5r)-6,7-dihydro-5h-pyrrolo(1,2-c)imidazol-5-yl)-3-fluoro-, Phosphate (1:1)
12. Isturisa (tn)
13. 4-[(5r)-6,7-dihydro-5h-pyrrolo[1,2-c]imidazol-5-yl]-3-fluorobenzonitrile Dihydrogen Phosphate
14. Chembl3707393
15. Schembl13837602
16. Dtxsid401027857
17. Osilodrostat Phosphate [mi]
18. Osilodrostat Phosphate (jan/usan)
19. Osilodrostat Phosphate [jan]
20. Hy-16276a
21. Akos040749099
22. Osilodrostat Phosphate [who-dd]
23. Da-76469
24. Osilodrostat Phosphate [orange Book]
25. Cs-0068058
26. D11062
27. Q27294304
28. 4-((5r)-6,7-dihydro-5h-pyrrolo(1,2-c)imidazol-5-yl)-3-fluorobenzonitrile Monophosphate
1. Lci699
2. Osilodrostat
3. Osilodrostat (lci699)
4. Osilodrostat Free Base
| Molecular Weight | 325.23 g/mol |
|---|---|
| Molecular Formula | C13H13FN3O4P |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 119 |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 387 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Isturisa is indicated for the treatment of endogenous Cushing's syndrome in adults.
H02CA02
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40908
Submission : 2025-01-07
Status : Active
Type : II







 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40908
Submission : 2025-01-07
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Alven Laboratories is a pharmaceutical company focused on the development, scale-up, and production of original and generic Active Pharmaceutical Ingredients (APIs). With expertise...
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
About the Company : Biophore, founded in 2007, develops and manufactures niche and complex pharmaceutical products. With USFDA- and EU-approved API facilities, a dedicated intermediates site and an R&...
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
About the Company : Maithri Drugs Pvt. Ltd. is a global supplier of Active Pharmaceutical Ingredients (APIs), serving pharmaceutical companies in 60+ countries. Its API portfolio spans antivirals, ant...
About the Company : BOC Sciences is a brand of BOCSCI Inc. We leverage our wide spectrum of business in the fields of development, manufacturing, marketing, and distribution to help you make best-info...

About the Company : DC Chemicals is a fine organics manufacturing facility based in Shanghai, P.R. China. The company offers a wide range of research chemicals and biochemicals, including novel life-s...

About the Company : Glixx Laboratories Inc (Glixx) is a global leading company specializing in biological research chemicals, providing services to meet the needs of biomedical research markets. With ...

About the Company : Hefei Home Sunshine Pharmaceutical Technology Co., Ltd.is a high-tech enterprise, which is an integration of R & D, producing and custom synthesis. The products quality is assured ...

About the Company : Sapphire Biotech, Inc. is dedicated to improving lives around the world through advancing global cancer care with innovative research and discovery methods. Our mission is to impro...

About the Company : Shanghai Yifei Biotechnology Co., Ltd., located in Shanghai, China's high-tech and educational hub, is a comprehensive biotechnology company specializing in product research and de...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Osilodrostat Phosphate is a small molecule drug candidate, which is currently being evaluated in Phase IV clinical studies for the treatment of Hypertension-associated with Hypercortisolaemia.
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 25, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Osilodrostat in Patients With Hypertension Caused by Hypercortisolaemia Due to Cushing's Syndrome
Details : Osilodrostat Phosphate is a small molecule drug candidate, which is currently being evaluated in Phase IV clinical studies for the treatment of Hypertension-associated with Hypercortisolaemia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 25, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Osilodrostat is a Other Small Molecule drug candidate, which is currently being evaluated in Phase II clinical studies for the treatment of unspecified medical condition.
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Undisclosed Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Recordati Rare Diseases
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 05, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Undisclosed
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Recordati Rare Diseases
Deal Size : Inapplicable
Deal Type : Inapplicable
Impact of 1 mg Osilodrostat Therapy on Mild Autonomous Cortisol Secretion (MACS)
Details : Osilodrostat is a Other Small Molecule drug candidate, which is currently being evaluated in Phase II clinical studies for the treatment of unspecified medical condition.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 05, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Isturisa (osilodrostat phosphate) is approved for the treatment of endogenous hypercortisolemia in adults with Cushing’s syndrome.
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Isturisa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 16, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Grants Isturisa (osilodrostat) Expanded Indication for Endogenous Hypercortisolemia
Details : Isturisa (osilodrostat phosphate) is approved for the treatment of endogenous hypercortisolemia in adults with Cushing’s syndrome.
Product Name : Isturisa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 16, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Isturisa (osilodrostat phosphate) is an oral inhibitor of CYP11B1, which catalyses the final step of cortisol synthesis in the adrenal glands. It is being evaluated for endogenous Cushing’s syndrome .
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Isturisa
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Recordati Rare Diseases to Present Data in Cushing’s Disease at ENDO 2024
Details : Isturisa (osilodrostat phosphate) is an oral inhibitor of CYP11B1, which catalyses the final step of cortisol synthesis in the adrenal glands. It is being evaluated for endogenous Cushing’s syndrome .
Product Name : Isturisa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 16, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Isturisa (osilodrostat phosphate) is an oral inhibitor of 11β-hydroxylase (CYP11B1), which catalyses the final step of cortisol synthesis in the adrenal glands. Isturisa® is available as 1 mg, 5 mg and 10 mg film-coated tablets.
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Isturisa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 31, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Isturisa (osilodrostat phosphate) is an oral inhibitor of 11β-hydroxylase (CYP11B1), which catalyses the final step of cortisol synthesis in the adrenal glands. Isturisa® is available as 1 mg, 5 mg and 10 mg film-coated tablets.
Product Name : Isturisa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 31, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ISTURISA® (osilodrostat), a cortisol synthesis inhibitor that works by inhibiting 11-beta-hydroxylase maintained normal mean urinary free cortisol (mUFC) long-term in patients with Cushing’s disease.
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Isturisa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 15, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : ISTURISA® (osilodrostat), a cortisol synthesis inhibitor that works by inhibiting 11-beta-hydroxylase maintained normal mean urinary free cortisol (mUFC) long-term in patients with Cushing’s disease.
Product Name : Isturisa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 15, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ISTURISA (osilodrostat), is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Isturisa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 08, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : ISTURISA (osilodrostat), is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
Product Name : Isturisa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 08, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
LINC 3 study demonstrated that Isturisa (osilodrostat) therapy provided long-term mean urinary free cortisol control and clinical improvements, with decreases inpatient weight and the severity of physical features, including hirsutism, that were maintained through to week 72.
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Isturisa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 13, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Phase III LINC 3 Study Demonstrates That ISTURISA® (osilodrostat) Improves Physical Features Asso...
Details : LINC 3 study demonstrated that Isturisa (osilodrostat) therapy provided long-term mean urinary free cortisol control and clinical improvements, with decreases inpatient weight and the severity of physical features, including hirsutism, that were maintain...
Product Name : Isturisa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 13, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Isturisa (osilodrostat) is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Isturisa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Isturisa (osilodrostat) is a cortisol synthesis inhibitor indicated for the treatment of adult patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
Product Name : Isturisa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The LINC 4 study demonstrated superiority of ISTURISA® (osilodrostat) over placebo in achieving cortisol control during the 12-week, double-blind, randomized phase (77% vs 8%, P<0.0001).
Lead Product(s): Osilodrostat Phosphate,Inapplicable
Therapeutic Area: Endocrinology Brand Name: Isturisa
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 29, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Osilodrostat Phosphate,Inapplicable
Therapeutic Area : Endocrinology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : The LINC 4 study demonstrated superiority of ISTURISA® (osilodrostat) over placebo in achieving cortisol control during the 12-week, double-blind, randomized phase (77% vs 8%, P<0.0001).
Product Name : Isturisa
Product Type : Miscellaneous
Upfront Cash : Inapplicable
March 29, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
71
PharmaCompass offers a list of Osilodrostat Phosphate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Osilodrostat Phosphate manufacturer or Osilodrostat Phosphate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Osilodrostat Phosphate manufacturer or Osilodrostat Phosphate supplier.
PharmaCompass also assists you with knowing the Osilodrostat Phosphate API Price utilized in the formulation of products. Osilodrostat Phosphate API Price is not always fixed or binding as the Osilodrostat Phosphate Price is obtained through a variety of data sources. The Osilodrostat Phosphate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Osilodrostat manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Osilodrostat, including repackagers and relabelers. The FDA regulates Osilodrostat manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Osilodrostat API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Osilodrostat manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Osilodrostat supplier is an individual or a company that provides Osilodrostat active pharmaceutical ingredient (API) or Osilodrostat finished formulations upon request. The Osilodrostat suppliers may include Osilodrostat API manufacturers, exporters, distributors and traders.
click here to find a list of Osilodrostat suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Osilodrostat DMF (Drug Master File) is a document detailing the whole manufacturing process of Osilodrostat active pharmaceutical ingredient (API) in detail. Different forms of Osilodrostat DMFs exist exist since differing nations have different regulations, such as Osilodrostat USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Osilodrostat DMF submitted to regulatory agencies in the US is known as a USDMF. Osilodrostat USDMF includes data on Osilodrostat's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Osilodrostat USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Osilodrostat suppliers with USDMF on PharmaCompass.
Osilodrostat Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Osilodrostat GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Osilodrostat GMP manufacturer or Osilodrostat GMP API supplier for your needs.
A Osilodrostat CoA (Certificate of Analysis) is a formal document that attests to Osilodrostat's compliance with Osilodrostat specifications and serves as a tool for batch-level quality control.
Osilodrostat CoA mostly includes findings from lab analyses of a specific batch. For each Osilodrostat CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Osilodrostat may be tested according to a variety of international standards, such as European Pharmacopoeia (Osilodrostat EP), Osilodrostat JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Osilodrostat USP).

