
Synopsis
Synopsis
0
EU WC
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
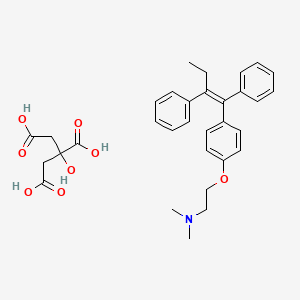

1. Citrate, Tamoxifen
2. Ici 46,474
3. Ici 46474
4. Ici 47699
5. Ici-46,474
6. Ici-46474
7. Ici-47699
8. Ici46,474
9. Ici46474
10. Ici47699
11. Nolvadex
12. Novaldex
13. Soltamox
14. Tamoxifen
15. Tomaxithen
16. Zitazonium
1. 54965-24-1
2. Zitazonium
3. Istubal
4. Kessar
5. Zemide
6. Tamoxifen (citrate)
7. Tamoxifen Citrate Salt
8. Nourytam
9. Tamofene
10. Tomaxasta
11. Ici 46474
12. Ici 46,474
13. Ici 46474 Citrate
14. Ici-46474
15. Nsc-180973
16. Nsc-757345
17. Tamoxifen Citrate (nolvadex)
18. 54965-24-1 (citrate)
19. 7frv7310n6
20. I.c.i.46474 Citrate
21. Nsc180973
22. Ncgc00024928-06
23. Cas-54965-24-1
24. Dsstox_cid_1301
25. (z)-2-(p-(1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethylethylamine Citrate (1:1)
26. Dsstox_rid_76068
27. Dsstox_gsid_21301
28. Ethanamine, 2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethyl-, (z)-,2-hydroxy-1,2,3-propanetricarboxylate (1:1)
29. Caditam
30. Farmifeno
31. Ginarsan
32. Jenoxifen
33. Ledertam
34. Nourytan
35. Noxitem
36. Oncotam
37. Tafoxen
38. Tamofen
39. Tamoxasta
40. Terimon
41. Zynoplex
42. Emblon
43. Genox
44. Nolgen
45. Noltam
46. Tamax
47. Tamoxifencitrate
48. Tamox-puren
49. (z)-2-(4-(1,2-diphenylbut-1-en-1-yl)phenoxy)-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate
50. 2-[4-[(z)-1,2-diphenylbut-1-enyl]phenoxy]-n,n-dimethylethanamine;2-hydroxypropane-1,2,3-tricarboxylic Acid
51. Ethanamine, 2-(4-(1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethyl, (z)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
52. Smr000677949
53. Z-tamoxifen Citrate
54. Sr-01000075523
55. Unii-7frv7310n6
56. Ccris 6718
57. Tamoxifeni Citras
58. Nolvadex (tn)
59. Soltamox (tn)
60. 2-{4-[(1z)-1,2-diphenylbut-1-en-1-yl]phenoxy}-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate (salt)
61. Prestwick_458
62. Einecs 259-415-2
63. Tamoxifen Citrate [usan:usp:jan]
64. Mfcd00058321
65. Tamoxifen (nolvadex)
66. Tamoxifen Citrate,(s)
67. Tamoxifen, Citrate Salt
68. Lopac-t-9262
69. Tamoxifen Z-isomer Citrate
70. Chembl786
71. Schembl6365
72. Mls001055370
73. Mls002154210
74. Spectrum1500557
75. Nolvadex (tn) (astrazeneca)
76. Tamoxifen Citrate [mi]
77. Tamoxifen Citrate [jan]
78. Dtxsid8021301
79. Tamoxifen Citrate (jp17/usp)
80. Tamoxifen Citrate [usan]
81. Hms500m20
82. Tamoxifen Citrate Salt, >=99%
83. Tamoxifen Citrate [vandf]
84. Tamoxifen Citrate [mart.]
85. Hms1568m14
86. Hms1921c13
87. Hms2092k17
88. Hms2095m14
89. Hms2232e08
90. Hms3263b08
91. Hms3675p04
92. Hms3712m14
93. Pharmakon1600-01500557
94. Tamoxifen Citrate [usp-rs]
95. Tamoxifen Citrate [who-dd]
96. Tamoxifen Citrate [who-ip]
97. Ex-a5777
98. Hydroxypropane-1,2,3-tricarboxylate
99. Tox21_110938
100. Tox21_202070
101. Tox21_300274
102. Tox21_501203
103. Ccg-39629
104. Nsc757345
105. S1238
106. S1972
107. Akos005111126
108. Tox21_110938_1
109. Cs-1852
110. Ks-5046
111. Lp01203
112. Tamoxifen Citrate [orange Book]
113. Tamoxifen Citrate [ep Monograph]
114. Ncgc00016206-01
115. Ncgc00016206-02
116. Ncgc00016206-03
117. Ncgc00016206-04
118. Ncgc00016206-05
119. Ncgc00016206-06
120. Ncgc00024928-02
121. Ncgc00024928-23
122. Ncgc00094450-01
123. Ncgc00094450-02
124. Ncgc00094450-03
125. Ncgc00094450-04
126. Ncgc00254000-01
127. Ncgc00259619-01
128. Ncgc00261888-01
129. Tamoxifen Citrate [usp Monograph]
130. Tamoxifeni Citras [who-ip Latin]
131. Hy-13757
132. Bcp0726000223
133. Tamoxifen Citrate - Cas 54965-24-1
134. Eu-0101203
135. Sw219368-1
136. T2510
137. (z)-2-(4-(1,2-diphenylbut-1-enyl)phenoxy)
138. A19582
139. D00966
140. T 9262
141. 965t241
142. Q-201776
143. Sr-01000075523-1
144. Sr-01000075523-3
145. Sr-01000075523-5
146. Sr-01000075523-6
147. Q27108378
148. Sr-01000075523-10
149. (z)-1-(4-dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene Citrate
150. Tamoxifen Citrate, European Pharmacopoeia (ep) Reference Standard
151. (z)-2-(4-(1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethylethanamine,citrate
152. (z)-2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethylethanamine Citrate
153. Tamoxifen Citrate, United States Pharmacopeia (usp) Reference Standard
154. (z)-2-[ P-(1,2-diphenyl-1-butenyl)phenoxy]- N, N-dimethylethylamine Citrate (1:1)
155. (z)-2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethyl-ethanamine Cirtrate (1:1)
156. Tamoxifen Citrate For Performance Test, European Pharmacopoeia (ep) Reference Standard
157. (2-{4-[1,2-diphenylbut-1-en-1-yl]phenoxy}ethyl)dimethylamine; 2-hydroxypropane-1,2,3-tricarboxylic Acid
158. (z)-(2-(4-(1,2-diphenylbut-1-enyl)phenoxy)ethyl)dimethylammonium Dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
159. (z)-2-(4-(1,2-diphenylbut-1-enyl)phenoxy)-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate
160. {2-[4-(1,2-diphenyl-1-buten-1-yl)phenoxy]ethyl}dimethylamine 2-hydroxy-1,2,3-propanetricarboxylate Salt
161. Ethanamine, 2-(4-((1z)-1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethyl-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
162. Ethanamine,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethyl-, (z)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
| Molecular Weight | 563.6 g/mol |
|---|---|
| Molecular Formula | C32H37NO8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 13 |
| Exact Mass | 563.25191714 g/mol |
| Monoisotopic Mass | 563.25191714 g/mol |
| Topological Polar Surface Area | 145 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 690 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Tamoxifen citrate |
| Drug Label | NOLVADEX (tamoxifen citrate) Tablets, a nonsteroidal antiestrogen, are for oral administration. NOLVADEX Tablets are available as:... |
| Active Ingredient | Tamoxifen citrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Teva; Actavis Labs Fl; Apotex; Teva Pharms; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Tamoxifen citrate |
| Drug Label | NOLVADEX (tamoxifen citrate) Tablets, a nonsteroidal antiestrogen, are for oral administration. NOLVADEX Tablets are available as:... |
| Active Ingredient | Tamoxifen citrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Teva; Actavis Labs Fl; Apotex; Teva Pharms; Mylan |
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Estrogen Antagonists
Compounds which inhibit or antagonize the action or biosynthesis of estrogenic compounds. (See all compounds classified as Estrogen Antagonists.)
Selective Estrogen Receptor Modulators
A structurally diverse group of compounds distinguished from ESTROGENS by their ability to bind and activate ESTROGEN RECEPTORS but act as either an agonist or antagonist depending on the tissue type and hormonal milieu. They are classified as either first generation because they demonstrate estrogen agonist properties in the ENDOMETRIUM or second generation based on their patterns of tissue specificity. (Horm Res 1997;48:155-63) (See all compounds classified as Selective Estrogen Receptor Modulators.)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Imlunestrant is a small molecule drug candidate, which is currently being evaluated in Phase II clinical studies for the treatment of Breast Neoplasms.
Lead Product(s): Imlunestrant,Goserelin Acetate,pre-filled syringe,Tamoxifen Citrate
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 17, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Imlunestrant,Goserelin Acetate,pre-filled syringe,Tamoxifen Citrate
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Imlunestrant is a small molecule drug candidate, which is currently being evaluated in Phase II clinical studies for the treatment of Breast Neoplasms.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 17, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
MK-5684 is a Other Small Molecule drug candidate, which is currently being evaluated in Phase II clinical studies for the treatment of Neoplasms.
Lead Product(s):
MK-5684,Fludrocortisone Acetate,Dexamethasone,Hydrocortisone,Fulvestrant,Exemestane,Megestrol Acetate,Medroxyprogesterone Acetate,Tamoxifen Citrate,
Therapeutic Area: Oncology
Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous Sponsor:
Undisclosed
Deal Size: Inapplicable
Upfront Cash: Inapplicable
Deal Type: Inapplicable
May 20, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fludrocortisone Acetate, Dexamethasone, Hydrocortisone, Fulvestrant, Exemestane
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
A Study of MK-5684 in People with Certain Solid Tumors (MK-5684-015/OMAHA-015)
Details : MK-5684 is a Other Small Molecule drug candidate, which is currently being evaluated in Phase II clinical studies for the treatment of Neoplasms.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
May 20, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Tamoxifen is a small molecule acts as an Estrogen receptor modulator, which is being investigated for the treatment of hormone receptor-positive breast cancer.
Lead Product(s): Tamoxifen Citrate,Inapplicable
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tamoxifen Citrate,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Kwality Pharma Gains on Securing SAHPRA Approval for Tamoxifen Tablet
Details : Tamoxifen is a small molecule acts as an Estrogen receptor modulator, which is being investigated for the treatment of hormone receptor-positive breast cancer.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 23, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Fezolinetant is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Hot Flashes.
Lead Product(s): Fezolinetant,Tamoxifen Citrate
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Fezolinetant,Tamoxifen Citrate
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Fezolinetant is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Hot Flashes.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 04, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
DARE-VVA1 (tamoxifen) is a small molecule vaginal insert acting as an Estrogen receptor modulator, which is being investigated for the treatment of moderate to severe dyspareunia.
Lead Product(s): Tamoxifen Citrate,Inapplicable
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: Undisclosed
Study Phase: Phase I/ Phase IIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 07, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Tamoxifen Citrate,Inapplicable
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Daré Bioscience Announces FDA Clearance of Investigational New Drug (IND) Application for DARE-VV...
Details : DARE-VVA1 (tamoxifen) is a small molecule vaginal insert acting as an Estrogen receptor modulator, which is being investigated for the treatment of moderate to severe dyspareunia.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 07, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Abemaciclib is a Other Small Molecule drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Breast Neoplasms.
Lead Product(s): Abemaciclib,Tamoxifen Citrate,Anastrozole,Letrozole,Exemestane,Luteinizing Hormone Releasing Hormone Agonist
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Miscellaneous
Sponsor: Eli Lilly
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 21, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Abemaciclib,Tamoxifen Citrate,Anastrozole,Letrozole,Exemestane,Luteinizing Hormone Releasing Hormone Agonist
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Eli Lilly
Deal Size : Inapplicable
Deal Type : Inapplicable
TRADE: Dose Escalation Tolerability of Abemaciclib in HR+ HER2- Early Stage Breast Cancer
Details : Abemaciclib is a Other Small Molecule drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Breast Neoplasms.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 21, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Inavolisib is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Breast Neoplasms.
Lead Product(s): Inavolisib,Pertuzumab,Trastuzumab,Tamoxifen Citrate,Anastrozole,Fulvestrant
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable June 08, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Inavolisib, Pertuzumab, Trastuzumab, Tamoxifen Citrate, Anastrozole
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Inavolisib is a Other Small Molecule drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Breast Neoplasms.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
June 08, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
DIACC3010 was well tolerated in monotherapy and potential biomarkers of pharmacological activity were seen in peripheral blood mononuclear cells and tumor tissues.
Lead Product(s): DIACC3010,Tamoxifen Citrate
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : DIACC3010,Tamoxifen Citrate
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : DIACC3010 was well tolerated in monotherapy and potential biomarkers of pharmacological activity were seen in peripheral blood mononuclear cells and tumor tissues.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
April 06, 2023

Details:
TQB3616 is a drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Breast Neoplasms.
Lead Product(s): TQB3616,Letrozole,Anastrozole,Tamoxifen Citrate
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Undisclosed
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable March 22, 2023

Lead Product(s) : TQB3616,Letrozole,Anastrozole,Tamoxifen Citrate
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : TQB3616 is a drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Breast Neoplasms.
Product Name : Undisclosed
Product Type : Undisclosed
Upfront Cash : Inapplicable
March 22, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
TOL2506 is a drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Breast Neoplasms.
Lead Product(s): TOL2506,Tamoxifen Citrate,Letrozole,Anastrozole,Exemestane
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Undisclosed
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 09, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : TOL2506,Tamoxifen Citrate,Letrozole,Anastrozole,Exemestane
Therapeutic Area : Oncology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : TOL2506 is a drug candidate, which is currently being evaluated in phase III clinical studies for the treatment of Breast Neoplasms.
Product Name : Undisclosed
Product Type : Undisclosed
Upfront Cash : Inapplicable
December 09, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
92
PharmaCompass offers a list of Tamoxifen Citrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Tamoxifen Citrate manufacturer or Tamoxifen Citrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Tamoxifen Citrate manufacturer or Tamoxifen Citrate supplier.
PharmaCompass also assists you with knowing the Tamoxifen Citrate API Price utilized in the formulation of products. Tamoxifen Citrate API Price is not always fixed or binding as the Tamoxifen Citrate Price is obtained through a variety of data sources. The Tamoxifen Citrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Tamoxifen manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Tamoxifen, including repackagers and relabelers. The FDA regulates Tamoxifen manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Tamoxifen API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Tamoxifen manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Tamoxifen supplier is an individual or a company that provides Tamoxifen active pharmaceutical ingredient (API) or Tamoxifen finished formulations upon request. The Tamoxifen suppliers may include Tamoxifen API manufacturers, exporters, distributors and traders.
click here to find a list of Tamoxifen suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Tamoxifen DMF (Drug Master File) is a document detailing the whole manufacturing process of Tamoxifen active pharmaceutical ingredient (API) in detail. Different forms of Tamoxifen DMFs exist exist since differing nations have different regulations, such as Tamoxifen USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Tamoxifen DMF submitted to regulatory agencies in the US is known as a USDMF. Tamoxifen USDMF includes data on Tamoxifen's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Tamoxifen USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Tamoxifen suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Tamoxifen Drug Master File in Japan (Tamoxifen JDMF) empowers Tamoxifen API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Tamoxifen JDMF during the approval evaluation for pharmaceutical products. At the time of Tamoxifen JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Tamoxifen suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Tamoxifen Drug Master File in Korea (Tamoxifen KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Tamoxifen. The MFDS reviews the Tamoxifen KDMF as part of the drug registration process and uses the information provided in the Tamoxifen KDMF to evaluate the safety and efficacy of the drug.
After submitting a Tamoxifen KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Tamoxifen API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Tamoxifen suppliers with KDMF on PharmaCompass.
A Tamoxifen CEP of the European Pharmacopoeia monograph is often referred to as a Tamoxifen Certificate of Suitability (COS). The purpose of a Tamoxifen CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Tamoxifen EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Tamoxifen to their clients by showing that a Tamoxifen CEP has been issued for it. The manufacturer submits a Tamoxifen CEP (COS) as part of the market authorization procedure, and it takes on the role of a Tamoxifen CEP holder for the record. Additionally, the data presented in the Tamoxifen CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Tamoxifen DMF.
A Tamoxifen CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Tamoxifen CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Tamoxifen suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Tamoxifen as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Tamoxifen API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Tamoxifen as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Tamoxifen and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Tamoxifen NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Tamoxifen suppliers with NDC on PharmaCompass.
Tamoxifen Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Tamoxifen GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Tamoxifen GMP manufacturer or Tamoxifen GMP API supplier for your needs.
A Tamoxifen CoA (Certificate of Analysis) is a formal document that attests to Tamoxifen's compliance with Tamoxifen specifications and serves as a tool for batch-level quality control.
Tamoxifen CoA mostly includes findings from lab analyses of a specific batch. For each Tamoxifen CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Tamoxifen may be tested according to a variety of international standards, such as European Pharmacopoeia (Tamoxifen EP), Tamoxifen JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Tamoxifen USP).

