
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Canada

0
South Africa

0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
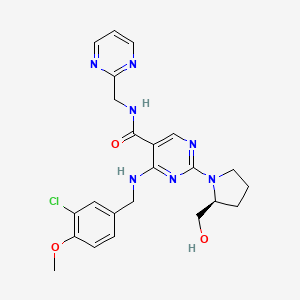

1. 4-((3-chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)-1-pyrrolidinyl)-n-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide
2. Stendra
1. 330784-47-9
2. Stendra
3. Spedra
4. Ta 1790
5. Ta-1790
6. (s)-4-((3-chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)pyrrolidin-1-yl)-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
7. Chebi:66876
8. Dr5s136ivo
9. (s)-2-(2-hydroxymethyl-1-pyrrolidinyl)-4-(3-chloro-4-methoxybenzylamino)-5-[(2-pyrimidinylmethyl)carbamoyl]pyrimidine
10. (s)-4-(3-chloro-4-methoxybenzylamino)-2-(2-hydroxymethylpyrrolidin-1-yl)-n-pyrimidin-2-ylmethyl-5-pyrimidinecarboxamide
11. (s)-4-[(3-chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-n-(2pyrimidinylmethyl)-5-pyrimidinecarboxamide
12. 4-[(3-chloro-4-methoxybenzyl)amino]-2-[(2s)-2-(hydroxymethyl)pyrrolidin-1-yl]-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
13. 4-[(3-chloro-4-methoxyphenyl)methylamino]-2-[(2s)-2-(hydroxymethyl)pyrrolidin-1-yl]-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
14. 4-((3-chloro-4-methoxybenzyl)amino)-2-((2s)-2-(hydroxymethyl)pyrrolidin-1-yl)-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
15. 5-pyrimidinecarboxamide, 4-(((3-chloro-4-methoxyphenyl)methyl)amino)-2-((2s)-2-(hydroxymethyl)-1-pyrrolidinyl)-n-(2-pyrimidinylmethyl)-
16. Stendra (tn)
17. Avanafil [usan:inn]
18. Unii-dr5s136ivo
19. Zepeed
20. Ine-5-carboxamide
21. Spedra (tn)
22. Avanafil [usan]
23. Avanafil (usan/inn)
24. Avanafil [inn]
25. Avanafil [mi]
26. Avanafil [vandf]
27. Avanafil [mart.]
28. Avanafil [who-dd]
29. Avanafil 100 Microg/ml In Acetonitrile:dimethylsulfoxide
30. Schembl118799
31. Avanafil [orange Book]
32. Gtpl7448
33. Chembl1963681
34. Amy1794
35. Dtxsid50186727
36. 4-((3-chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)-1-pyrrolidinyl)-n-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide
37. Bdbm50036629
38. Cs1566
39. Mfcd11977961
40. S4019
41. Ta1790
42. Zinc11677857
43. Akos024462448
44. Ccg-229896
45. Db06237
46. Vi-0162
47. Ncgc00386241-01
48. As-20106
49. Hy-18252
50. Sw219217-1
51. D03217
52. Ab01565827_02
53. J-019006
54. Q2873270
55. Brd-k65781196-001-01-4
56. (s)-2-(2-hydroxymethyl-1-pyrrolidinyl)-4-(3-chloro-4-methoxybenzylamino)-5-[n-(2-pyrimidylmethyl)carbamoyl]-pyrimidine
57. (s)-4-[(3-chlor-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1- Pyrrolidinyl]-n-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamid
58. 4-[(3-chloranyl-4-methoxy-phenyl)methylamino]-2-[(2s)-2-(hydroxymethyl)pyrrolidin-1-yl]-n-(pyrimidin-2-ylmethyl)pyrimid
59. 4-[(3-chloranyl-4-methoxy-phenyl)methylamino]-2-[(2s)-2-(hydroxymethyl)pyrrolidin-1-yl]-n-(pyrimidin-2-ylmethyl)pyrimid Ine-5-carboxamide
60. Avanafil; (s)-4-chloro-6-((3-chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)pyrrolidin-1-yl)-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
61. E6l
| Molecular Weight | 483.9 g/mol |
|---|---|
| Molecular Formula | C23H26ClN7O3 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 9 |
| Exact Mass | 483.1785654 g/mol |
| Monoisotopic Mass | 483.1785654 g/mol |
| Topological Polar Surface Area | 125 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 642 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Stendra |
| PubMed Health | Avanafil (By mouth) |
| Drug Classes | Erectile Dysfunction Agent |
| Drug Label | STENDRA (avanafil) is a selective inhibitor of cGMP-specific PDE5.Avanafil is designated chemically as (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide and has the following... |
| Active Ingredient | Avanafil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Vivus |
| 2 of 2 | |
|---|---|
| Drug Name | Stendra |
| PubMed Health | Avanafil (By mouth) |
| Drug Classes | Erectile Dysfunction Agent |
| Drug Label | STENDRA (avanafil) is a selective inhibitor of cGMP-specific PDE5.Avanafil is designated chemically as (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide and has the following... |
| Active Ingredient | Avanafil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Vivus |
Avanafil is indicated for the treatment of erectile dysfunction.
FDA Label
Treatment of erectile dysfunction in adult men.
In order for Spedra to be effective, sexual stimulation is required.
Avanafil is a strong competitive inhibitor of phosphodiesterase 5 (PDE5) with a demonstrated _in vitro_ IC50 of 5.2 nM. Its inhibitory effects on PDE5 are 100-fold more potent than on PDE6 and >1000-fold more potent than on other PDE enzymes, meaning it is less likely to cause visual disturbances and cardiovascular adverse effects when compared with less selective PDE5 inhibitors such as [sildenafil] and [vardenafil]. It has a relatively quick onset of action allowing for administration as early as 15 minutes prior to sexual activity. PDE5 inhibitors like avanafil can cause significant drug interactions when administered alongside certain antihypertensive agents (e.g. alpha blockers, substantial amounts of alcohol). PDE5 inhibitors have also been associated with the development of non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition that typically presents as sudden loss of vision in one or both eyes and appears to be more common in patients with a "crowded" optic disc. Patients presenting with any degree of vision loss should immediately discontinue use of all PDE5 inhibitors and seek medical attention. In some jurisdictions, a history of NAION or other degenerative retinal disorders is considered a contraindication to avanafil therapy.
G04BE10
G04BE10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BE - Drugs used in erectile dysfunction
G04BE10 - Avanafil
Absorption
Avanafil is rapidly absorbed following oral administration (Tmax of 30-45 minutes) and appears to have low to moderate oral bioavailability, though formal studies have not been conducted. Administration with a meal results in a mean delay in Tmax of 1.12 to 1.25 hours, a 39% mean reduction in Cmax, and a negligible effect on AUC.
Route of Elimination
Following oral administration, avanafil is extensively metabolized. Approximately 62% of a given dose is excreted as metabolites in the feces and approximately 21% as metabolites in the urine.
Volume of Distribution
The apparent volume of distribution of avanafil is 47 to 83 L.
Avanafil is extensively metabolized, primarily by CYP3A4 and to a lesser extent by CYP2C9. There are two major metabolites formed, M4 and M16, which exist in the plasma at concentrations 23% and 29% that of the parent compound, respectively. The M16 metabolite lacks pharmacologic effect, but the M4 metabolite has an inhibitory potency for PDE5 18% that of avanafil and accounts for approximately 4% of the observed pharmacologic activity of avanafil.
Studies have demonstrated variability in the terminal elimination half-life of avanafil, with estimates ranging between 5 - 17 hours.
Avanafil inhibits the cGMP-specific phosphodiesterase type 5 (PDE5) which is responsible for the degradation of cGMP in the corpus cavernosum located around the penis. Sexual arousal results in the local release of nitric oxide, which in turn stimulates the enzyme guanylate cyclase to produce cGMP. Elevated levels of cGMP result in local smooth muscle relaxation and increased blood flow to the penis (i.e. an erection). As PDE5 inhibitors like avanafil require the endogenous release of nitric oxide in order to exert their pharmacologic effect, they have no effect on the user in the absence of sexual stimulation/arousal.
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 28732
Submission : 2014-10-07
Status : Active
Type : II
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
Century has been an API manufacturer for over 40 years & is the partner of choice for multipurpose custom manufacturing projects.
 Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.

GDUFA
DMF Review : Reviewed
Rev. Date : 2016-02-05
Pay. Date : 2015-12-03
DMF Number : 30024
Submission : 2015-12-15
Status : Active
Type : II

NDC Package Code : 69037-0019
Start Marketing Date : 2012-04-27
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 36175
Submission : 2021-09-16
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 24849
Submission : 2011-05-19
Status : Active
Type : II





 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Details:
Undisclosed
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Undisclosed
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 20, 2015
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Efficacy and Tolerability Study of Avanafil in Russia
Details : Undisclosed
Product Name : Undisclosed
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 20, 2015
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
STENDRA (avanafil), an oral phosphodiesterase 5 (PDE5) inhibitor for treatment of erectile dysfunction, provides positive results from its OTC draft label comprehension study for its ED drug demonstrated understanding of the messages therein.
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Stendra
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Auxilium Pharmaceuticals
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 19, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Auxilium Pharmaceuticals
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : STENDRA (avanafil), an oral phosphodiesterase 5 (PDE5) inhibitor for treatment of erectile dysfunction, provides positive results from its OTC draft label comprehension study for its ED drug demonstrated understanding of the messages therein.
Product Name : Stendra
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
April 19, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The new partnership, which replaces a previous agreement with Vivus, will transfer manufacturing of the STENDRA (avanafil) tablets, to the United States, and is expected to provide both cost savings and increase in gross margin.
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Stendra
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Undisclosed
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership January 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Undisclosed
Deal Type : Partnership
Details : The new partnership, which replaces a previous agreement with Vivus, will transfer manufacturing of the STENDRA (avanafil) tablets, to the United States, and is expected to provide both cost savings and increase in gross margin.
Product Name : Stendra
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
January 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Petros Pharmaceuticals intends to use the net proceeds from this offering for expansion of Petros Pharmaceuticals' men's health platform including Stendra and for working capital and general corporate purposes.
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Stendra
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Katalyst Securities
Deal Size: $10.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering November 30, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Katalyst Securities
Deal Size : $10.0 million
Deal Type : Public Offering
Petros Pharmaceuticals Announces Closing of $10 Million Offering
Details : Petros Pharmaceuticals intends to use the net proceeds from this offering for expansion of Petros Pharmaceuticals' men's health platform including Stendra and for working capital and general corporate purposes.
Product Name : Stendra
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
November 30, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Petros Pharmaceuticals intends to use the net proceeds from this offering for expansion of its men's health platform which includes enhancing the position of STENDRA, and for working capital and general corporate purposes.
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Stendra
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Katalyst Securities
Deal Size: $5.7 million Upfront Cash: Undisclosed
Deal Type: Public Offering October 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Katalyst Securities
Deal Size : $5.7 million
Deal Type : Public Offering
Petros Pharmaceuticals Announces Closing of $5.7 Million Registered Direct Offering
Details : Petros Pharmaceuticals intends to use the net proceeds from this offering for expansion of its men's health platform which includes enhancing the position of STENDRA, and for working capital and general corporate purposes.
Product Name : Stendra
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
October 19, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Stendra (avanafil), originally launched by Auxilium Pharmaceuticals prior to that company's sale to Endo Pharmaceuticals, is an oral phosphodiesterase 5 (PDE5) inhibitor for the treatment of erectile dysfunction.
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Stendra
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 27, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
FDA Meeting to Petros Pharma Discuss Label Expansion of Drug STENDRA®
Details : Stendra (avanafil), originally launched by Auxilium Pharmaceuticals prior to that company's sale to Endo Pharmaceuticals, is an oral phosphodiesterase 5 (PDE5) inhibitor for the treatment of erectile dysfunction.
Product Name : Stendra
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 27, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The Company has initiated a Phase 2 study, which is more significant & Label Comprehension Study that will include low health literacy individuals as part of a multi-step process to develop the behavioral study evidence for FDA to evaluate Stendra for possible OTC designation.
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Stendra
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable July 13, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Petros Completes Pilot Label Comprehension Study to Initiate Process for Stendra® Prescription Er...
Details : The Company has initiated a Phase 2 study, which is more significant & Label Comprehension Study that will include low health literacy individuals as part of a multi-step process to develop the behavioral study evidence for FDA to evaluate Stendra for po...
Product Name : Stendra
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
July 13, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Further expanding the partnership, the companies announce the availability of additional dosages of STENDRA in 50mg, 100mg and 200mg tablets, all of which are now accessible through the Hims platform.
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Stendra
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Hims & Hers Health
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement March 11, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Hims & Hers Health
Deal Size : Undisclosed
Deal Type : Agreement
Details : Further expanding the partnership, the companies announce the availability of additional dosages of STENDRA in 50mg, 100mg and 200mg tablets, all of which are now accessible through the Hims platform.
Product Name : Stendra
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
March 11, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Petros' cornerstone product would be Metuchen's Stendra® for erectile dysfunction. Petros' pipeline would include Metuchen's recently in-licensed product H-100 for Peyronie's disease, and it would include a business development program exploring various men's health products.
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Psychiatry/Psychology Brand Name: Stendra
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Neurotrope
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Merger May 18, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Neurotrope
Deal Size : Undisclosed
Deal Type : Merger
Details : Petros' cornerstone product would be Metuchen's Stendra® for erectile dysfunction. Petros' pipeline would include Metuchen's recently in-licensed product H-100 for Peyronie's disease, and it would include a business development program exploring variou...
Product Name : Stendra
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
May 18, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Undisclosed
Lead Product(s): Avanafil,Inapplicable
Therapeutic Area: Undisclosed Brand Name: Undisclosed
Study Phase: Phase IVProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 10, 2014

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Avanafil,Inapplicable
Therapeutic Area : Undisclosed
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Safety Study Looking at the Effects of Stendra on Vision
Details : Undisclosed
Product Name : Undisclosed
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
January 10, 2014

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
21
PharmaCompass offers a list of Avanafil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Avanafil manufacturer or Avanafil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Avanafil manufacturer or Avanafil supplier.
PharmaCompass also assists you with knowing the Avanafil API Price utilized in the formulation of products. Avanafil API Price is not always fixed or binding as the Avanafil Price is obtained through a variety of data sources. The Avanafil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A stendra manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of stendra, including repackagers and relabelers. The FDA regulates stendra manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. stendra API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of stendra manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A stendra supplier is an individual or a company that provides stendra active pharmaceutical ingredient (API) or stendra finished formulations upon request. The stendra suppliers may include stendra API manufacturers, exporters, distributors and traders.
click here to find a list of stendra suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A stendra DMF (Drug Master File) is a document detailing the whole manufacturing process of stendra active pharmaceutical ingredient (API) in detail. Different forms of stendra DMFs exist exist since differing nations have different regulations, such as stendra USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A stendra DMF submitted to regulatory agencies in the US is known as a USDMF. stendra USDMF includes data on stendra's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The stendra USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of stendra suppliers with USDMF on PharmaCompass.
A stendra written confirmation (stendra WC) is an official document issued by a regulatory agency to a stendra manufacturer, verifying that the manufacturing facility of a stendra active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting stendra APIs or stendra finished pharmaceutical products to another nation, regulatory agencies frequently require a stendra WC (written confirmation) as part of the regulatory process.
click here to find a list of stendra suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing stendra as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for stendra API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture stendra as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain stendra and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a stendra NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of stendra suppliers with NDC on PharmaCompass.
stendra Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of stendra GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right stendra GMP manufacturer or stendra GMP API supplier for your needs.
A stendra CoA (Certificate of Analysis) is a formal document that attests to stendra's compliance with stendra specifications and serves as a tool for batch-level quality control.
stendra CoA mostly includes findings from lab analyses of a specific batch. For each stendra CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
stendra may be tested according to a variety of international standards, such as European Pharmacopoeia (stendra EP), stendra JP (Japanese Pharmacopeia) and the US Pharmacopoeia (stendra USP).
