
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Acomplia
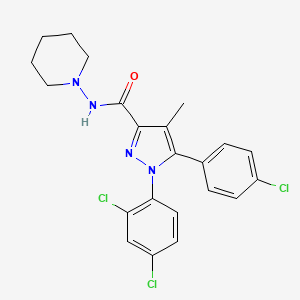
2. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1h-pyrazole-3-carboxamide Hydrochloride
3. Rimonabant Hydrochloride
4. Sr 141716
5. Sr 141716a
6. Sr-141716a
7. Sr141716
8. Sr141716a
9. Zimulti
1. 168273-06-1
2. Acomplia
3. Zimulti
4. 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-(piperidin-1-yl)-1h-pyrazole-3-carboxamide
5. Sr141716
6. Rimoslim
7. Sr 141716
8. Sr141716a
9. Sr-141716a
10. A 281
11. Rimonabant Free Base
12. 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-piperidin-1-ylpyrazole-3-carboxamide
13. Sr-141716
14. Rml78en3xe
15. Chembl111
16. Rimonabant (sr141716)
17. Chebi:34967
18. Ncgc00164572-01
19. Sr-14171
20. [3h]sr141716a
21. 158681-13-1 (hcl)
22. [3h]rimonabant
23. Dsstox_cid_26453
24. Dsstox_rid_81627
25. Dsstox_gsid_46453
26. 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1h-pyrazole-3-carboxylic Acid Piperidin-1-ylamide
27. Acomplia (tn)
28. Rimonabant [inn]
29. Smr003500713
30. Cas-168273-06-1
31. Unii-rml78en3xe
32. Rimonabant (jan/usan/inn)
33. Rimonabant [usan:inn:jan]
34. 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-1-piperidinyl-1h-pyrazole-3-carboxamide
35. Rimonabant- Bio-x
36. 1h-pyrazole-3-carboxamide, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-1-piperidinyl-
37. Sr141,716a
38. Rimonabant [mi]
39. Rimonabant [jan]
40. 1h-pyrazole-3-carboxamide, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-1-piperidinyl-, Monohydrochloride
41. Rimonabant - From Sample
42. Rimonabant [usan]
43. Rimonabant(sr141716)
44. Rimonabant [mart.]
45. 5-(p-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-piperidinopyrazole-3-carboxamide
46. Rimonabant [who-dd]
47. Schembl38637
48. Gtpl743
49. Mls004774043
50. Mls006011772
51. Rimonabant [ema Epar]
52. Dtxsid3046453
53. Bdbm21278
54. Ex-a688
55. Hms3604m05
56. Hms3657o15
57. Bcp07803
58. Zinc1540228
59. Tox21_112200
60. Ac-731
61. Bbl030198
62. Mfcd04034714
63. Nsc791533
64. S3021
65. Stk642500
66. Akos005266728
67. Tox21_112200_1
68. Am84578
69. Ccg-269385
70. Cs-0645
71. Db06155
72. Nsc-791533
73. Sb19549
74. 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-(1-piperidyl)pyrazole-3-carboxamide
75. 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-piperidino-1h-pyrazole-3-carboxamide
76. Ncgc00164572-02
77. As-37280
78. Br164345
79. Hy-14136
80. Ac-163720
81. Db-011649
82. B1429
83. Ft-0631194
84. R0205
85. Sw220167-1
86. 81r131
87. D05731
88. Ab01566860_01
89. A810956
90. L000572
91. Q412529
92. Sr-01000884001
93. J-010440
94. Sr-01000884001-1
95. (n-(piperidin-1-yl)-5-(4-chlorophenyl)-4-methyl-1h-pyrazole-3-carboxamide
96. 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-(1-piperidinyl)-3-pyrazolecarboxamide
97. 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-piperidin-1-yl-pyrazole-3-carboxamide
98. N-piperidino-5-(4-chlorophenyl)-1(2,4-dichlorophenyl)-4-methyl-pyrazole-3-carboxamide
99. N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazolecarboxamide
100. 1h-pyrazole-3-carboxamide,5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-n-1-piperidinyl-
101. 5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-1h-pyrazole-3-carboxylic Acid Piperidin-1-ylamide
102. Ay6
| Molecular Weight | 463.8 g/mol |
|---|---|
| Molecular Formula | C22H21Cl3N4O |
| XLogP3 | 6.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 462.078094 g/mol |
| Monoisotopic Mass | 462.078094 g/mol |
| Topological Polar Surface Area | 50.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 583 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For use in conjunction with diet and exercise for patients with a body mass index greater than 30 kg/m2, or patients wih a BMI greater than 27 kg/m2 with associated risk factors, such as type 2 diabetes or dyslipidaemia.
As an adjunct to diet and exercise for the treatment of obese patients (BMI 30 kg/m2), or overweight patients (BMI 27 kg/m2) with associated risk factor(s), such as type 2 diabetes or dyslipidaemia (see section 5. 1).
As an adjunct to diet and exercise for the treatment of obese patients (BMI 30 kg/m2), or overweight patients (BMI 27 kg/m2) with associated risk factor(s), such as type 2 diabetes or dyslipidaemia (see section 5. 1).
In the RIO-North America trial, 3040 patients were randomized to receive either placebo or one of two doses of rimonabant (5 mg or 20 mg per day). Patients taking 20 mg rimonabant had significant weigh loss, decrease in waist circumference, improved insulin sensitivity, and increases in HDL cholesterol, compared to patients on placebo.
Anti-Obesity Agents
Agents that increase energy expenditure and weight loss by neural and metabolic regulation. (See all compounds classified as Anti-Obesity Agents.)
Cannabinoid Receptor Antagonists
Compounds that inhibit or block the activity of CANNABINOID RECEPTORS. (See all compounds classified as Cannabinoid Receptor Antagonists.)
A08AX01
A08AX01
A - Alimentary tract and metabolism
A08 - Antiobesity preparations, excl. diet products
A08A - Antiobesity preparations, excl. diet products
A08AX - Other antiobesity drugs
A08AX01 - Rimonabant
Absorption
Undetermined
Hepatic, CYP3A4 involved.
6 to 9 days with normal BMI and 16 days if BMI is greater than 30
Rimonabant is a specific CB1 cannabinoid receptor antagonist. There is considerable evidence that the endocannabinoid (endogenous cannabinoid) system plays a significant role in appetitive drive and associated behaviours. It is therefore reasonable to hypothesize that the attenuation of the activity of this system would have therapeutic benefit in treating disorders that might have a component of excess appetitive drive or over-activity of the endocannabinoid system, such as obesity, ethanol and other drug abuse, and a variety of central nervous system and other disorders.
ABOUT THIS PAGE
49
PharmaCompass offers a list of Rimonabant API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Rimonabant manufacturer or Rimonabant supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Rimonabant manufacturer or Rimonabant supplier.
PharmaCompass also assists you with knowing the Rimonabant API Price utilized in the formulation of products. Rimonabant API Price is not always fixed or binding as the Rimonabant Price is obtained through a variety of data sources. The Rimonabant Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Rimoslim manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Rimoslim, including repackagers and relabelers. The FDA regulates Rimoslim manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Rimoslim API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Rimoslim manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Rimoslim supplier is an individual or a company that provides Rimoslim active pharmaceutical ingredient (API) or Rimoslim finished formulations upon request. The Rimoslim suppliers may include Rimoslim API manufacturers, exporters, distributors and traders.
click here to find a list of Rimoslim suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Rimoslim DMF (Drug Master File) is a document detailing the whole manufacturing process of Rimoslim active pharmaceutical ingredient (API) in detail. Different forms of Rimoslim DMFs exist exist since differing nations have different regulations, such as Rimoslim USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Rimoslim DMF submitted to regulatory agencies in the US is known as a USDMF. Rimoslim USDMF includes data on Rimoslim's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Rimoslim USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Rimoslim suppliers with USDMF on PharmaCompass.
Rimoslim Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Rimoslim GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Rimoslim GMP manufacturer or Rimoslim GMP API supplier for your needs.
A Rimoslim CoA (Certificate of Analysis) is a formal document that attests to Rimoslim's compliance with Rimoslim specifications and serves as a tool for batch-level quality control.
Rimoslim CoA mostly includes findings from lab analyses of a specific batch. For each Rimoslim CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Rimoslim may be tested according to a variety of international standards, such as European Pharmacopoeia (Rimoslim EP), Rimoslim JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Rimoslim USP).
