
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
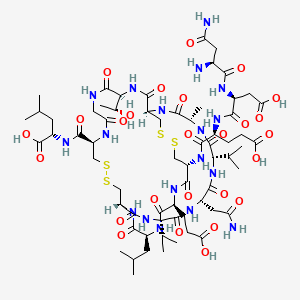

1. Sp-304
2. Trulance
1. Trulance
2. Guanilib
3. 467426-54-6
4. 467426-54-6 (free Base)
5. Plecanatide [usan:inn]
6. Unii-7ik8z952ok
7. Sp-304 (synergy)
8. 7ik8z952ok
9. Gtpl9069
10. Chembl2103867
11. Hsdb 8405
12. Dtxsid60196933
13. Glxc-26182
14. Ex-a4131
15. Db13170
16. [3-glutamic Acid(d>e)]human Uroguanylin
17. (3-glutamic Acid(d>e))human Uroguanylin (ugn)
18. L-asparaginyl-l-alpha-aspartyl-l-alpha-glutamyl-l-cysteinyl-l-alpha-glutamyl-l-leucyl-l-cysteinyl-l-valyl-l-asparaginyl-l-valyl-l-alanyl-l-cysteinyl-l-threonylglycyl-l-cysteinyl-l-leucine Cyclic (4-->12),(7-->15)-bis(disulfide)
19. L-asparaginyl-l-alpha-aspartyl-l-alpha-glutamyl-l-cysteinyl-l-alpha-glutamyl-l-leucyl-l-cysteinyl-l-valyl-l-asparaginyl-l-valyl-l-alanyl-l-cysteinyl-l-threonylglycyl-l-cysteinyl-l-leucine Cyclic (4adagger?2),(7adagger?5)-bis(disulfide)
20. L-leucine, L-asparaginyl-l-alpha-aspartyl-l-alpha-glutamyl-l-cysteinyl-l-alpha-glutamyl-l-leucyl-l- Cysteinyl-l-valyl-l-asparaginyl-l-valyl-l-alanyl-l-cysteinyl-l-threonylglycyl-l-cysteinyl-, Cyclic (4->12),(7->15)-bis(disulfide)
| Molecular Weight | 1681.9 g/mol |
|---|---|
| Molecular Formula | C65H104N18O26S4 |
| XLogP3 | -8.2 |
| Hydrogen Bond Donor Count | 23 |
| Hydrogen Bond Acceptor Count | 31 |
| Rotatable Bond Count | 28 |
| Exact Mass | 1680.6252002 g/mol |
| Monoisotopic Mass | 1680.6252002 g/mol |
| Topological Polar Surface Area | 819 Ų |
| Heavy Atom Count | 113 |
| Formal Charge | 0 |
| Complexity | 3490 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 16 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Guanylyl Cyclase C Agonists; Gastrointestinal Agents
National Library of Medicine's Medical Subject Headings. Plecanatide. Online file (MeSH, 2018). Available from, as of March 7, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Plecanatide is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 7, 2018: https://clinicaltrials.gov/
Trulance is indicated in adults for the treatment of: chronic idiopathic constipation (CIC); Irritable bowel syndrome with constipation (IBS-C). /Included in US product label/
NIH; DailyMed. Current Medication Information for Trulance Immediate Release (Plecanatide) Tablet (Updated: February 7, 2018). Available from, as of March 21, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1766455-79a2-409f-9325-0e3d6743acc7
/EXPL THER/ /The aim of this study was/ to evaluate the effect of orally administered plecanatide or dolcanatide, analogs of uroguanylin, on amelioration of colitis in murine models. The cyclic guanosine monophosphate (cGMP) stimulatory potency of plecanatide and dolcanatide was measured using a human colon carcinoma T84 cell-based assay. For animal studies all test agents were formulated in phosphate buffered saline. Sulfasalazine or 5-amino salicylic acid (5-ASA) served as positive controls. Effect of oral treatment with test agents on amelioration of acute colitis induced either by dextran sulfate sodium (DSS) in drinking water or by rectal instillation of trinitrobenzene sulfonic (TNBS) acid, was examined in BALB/c and/or BDF1 mice. Additionally, the effect of orally administered plecanatide on the spontaneous colitis in T-cell receptor alpha knockout (TCRa(-/-)) mice was also examined. Amelioration of colitis was assessed by monitoring severity of colitis, disease activity index and by histopathology. Frozen colon tissues were used to measure myeloperoxidase activity. Plecanatide and dolcanatide are structurally related analogs of uroguanylin, which is an endogenous ligand of guanylate cyclase-C (GC-C). As expected from the agonists of GC-C, both plecanatide and dolcanatide exhibited potent cGMP-stimulatory activity in T84 cells. Once-daily treatment by oral gavage with either of these analogs (0.05-0.5 mg/kg) ameliorated colitis in both DSS and TNBS-induced models of acute colitis, as assessed by body weight, reduction in colitis severity (P < 0.05) and disease activity index (P < 0.05). Amelioration of colitis by either of the drug candidates was comparable to that achieved by orally administered sulfasalazine or 5-ASA. Plecanatide also effectively ameliorated colitis in TCRa(-/-) mice, a model of spontaneous colitis. As dolcanatide exhibited higher resistance to proteolysis in simulated gastric and intestinal juices, it was selected for further studies. This is the first-ever study reporting the therapeutic utility of GC-C agonists as a new class of orally delivered and mucosally active drug candidates for the treatment of inflammatory bowel diseases.
PMID:26558155 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4635161 Shailubhai K et al; World J Gastrointest Pharmacol Ther 6 (4): 213-22 (2015)
/BOXED WARNING/ WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS. Trulance is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile mice administration of a single oral dose of plecanatide caused deaths due to dehydration. Avoid use of Trulance in patients 6 years to less than 18 years of age. The safety and effectiveness of Trulance have not been established in patients less than 18 years of age.
NIH; DailyMed. Current Medication Information for Trulance Immediate Release (Plecanatide) Tablet (Updated: February 7, 2018). Available from, as of March 21, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1766455-79a2-409f-9325-0e3d6743acc7
Since systemic absorption of plecanatide and its active metabolite is negligible following oral administration, the drug is not expected to result in fetal exposure if administered to pregnant women. However, available data on use of plecanatide in pregnant women are insufficient to inform fetal risk.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
It is not known whether plecanatide is distributed into human milk, affects milk production, or affects the breast-fed infant. Systemic absorption of plecanatide and its active metabolite is negligible following oral administration. It is not known whether the negligible systemic absorption observed in adults will result in clinically important exposure in breast-fed infants. The benefits of breast-feeding and the importance of plecanatide to the woman should be considered along with potential adverse effects on the breast-fed infant from the drug or from the underlying maternal condition. Exposure of infants to plecanatide could result in serious adverse effects.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
Trulance is contraindicated in: Patients less than 6 years of age due to the risk of serious dehydration. Patients with known or suspected mechanical gastrointestinal obstruction.
NIH; DailyMed. Current Medication Information for Trulance Immediate Release (Plecanatide) Tablet (Updated: February 7, 2018). Available from, as of March 21, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1766455-79a2-409f-9325-0e3d6743acc7
For more Drug Warnings (Complete) data for Plecanatide (10 total), please visit the HSDB record page.
Plecanatide stimulates intestinal fluid secretions in the gastrointestinal tract to support regular bowel function. Plecanatide, taken orally once daily, works locally in the upper GI tract to stimulate secretion of intestinal fluid and support regular bowel function.
Food Effect Subjects who received either a low-fat, low calorie (LF-LC) meal or a high fat, high calorie (HF-HC) meal reported looser stools than fasted subjects up to 24 hours after a single dose of 9 mg (3 times the recommended dose). In clinical studies, Plecanatide was administered with or without food.
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
Guanylyl Cyclase C Agonists
Compunds that bind to and activate GUANYLYL CYCLASE-C RECEPTORS. (See all compounds classified as Guanylyl Cyclase C Agonists.)
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AX - Other drugs for constipation
A06AX07 - Plecanatide
Absorption
Plecanatide is minimally absorbed with negligible systemic availability following oral administration. Concentrations of plecanatide and its active metabolite in plasma are below the limit of quantitation after an oral dose of 3 mg. Therefore, standard pharmacokinetic parameters such as AUC, maximum concentration (Cmax), and half-life (t) cannot be calculated.
Route of Elimination
No excretion studies have been conducted in humans. Plecanatide and its active metabolite are not measurable in plasma following administration of the recommended clinical doses.
Volume of Distribution
Concentrations of plecanatide and its active metabolite in plasma are below the limit of quantitation after an oral dose of 3 mg. Therefore, the volume of distribution can not be calculated.
Clearance
No excretion studies have been conducted in humans.
Given that plecanatide concentrations following clinically relevant oral doses were not measurable, plecanatide is expected to be minimally distributed in tissues. Oral plecanatide was localized to the GI tract where it exerted its effects as a guanylate cyclase-C (GC-C) agonist with negligible systemic exposure. Plecanatide exhibited little to no binding to human serum albumin or human a-1-acid glycoprotein.
NIH; DailyMed. Current Medication Information for Trulance Immediate Release (Plecanatide) Tablet (Updated: February 7, 2018). Available from, as of March 21, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1766455-79a2-409f-9325-0e3d6743acc7
In a crossover study, 24 healthy subjects were given a single dose of Trulance 9 mg (3 times the recommended dose) in 3 different states: fasted; following a low-fat, low-calorie meal (LF-LC; approximately 350 calories: 17% from fat, 66% from carbohydrate, and 17% from protein); and following a high-fat, high-calorie meal (HF-HC; approximately 1000 calories: 60% from fat, 25% from carbohydrate, and 15% from protein). Plecanatide was detected in 1 subject (fasted state) at 0.5 and 1 hour post dose. Plecanatide concentrations were below the limit of quantitation for all other time points and for all other subjects. The active metabolite was not detected in any subject.
NIH; DailyMed. Current Medication Information for Trulance Immediate Release (Plecanatide) Tablet (Updated: February 7, 2018). Available from, as of March 21, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1766455-79a2-409f-9325-0e3d6743acc7
Plecanatide was minimally absorbed with negligible systemic availability following oral administration. Concentrations of plecanatide and its active metabolite in plasma were below the limit of quantitation in the majority of analyzed plasma samples after an oral Trulance dose of 3 mg. Therefore, standard pharmacokinetic parameters such as AUC, maximum concentration (Cmax), and half-life could not be calculated.
NIH; DailyMed. Current Medication Information for Trulance Immediate Release (Plecanatide) Tablet (Updated: February 7, 2018). Available from, as of March 21, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1766455-79a2-409f-9325-0e3d6743acc7
Plecanatide is metabolized in the GI tract to an active metabolite by loss of the terminal leucine moiety. Both plecanatide and the metabolite are proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids.
Plecanatide was metabolized in the GI tract to an active metabolite by loss of the terminal leucine moiety. Both plecanatide and the metabolite were proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids.
NIH; DailyMed. Current Medication Information for Trulance Immediate Release (Plecanatide) Tablet (Updated: February 7, 2018). Available from, as of March 21, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1766455-79a2-409f-9325-0e3d6743acc7
half-life (t) cannot be calculated due to negligible systemic absorbance
Guanylate cyclase C (GC-C) agonist Plecanatide and its active metabolite bind to GC-C and act locally on the luminal surface of intestinal epithelial cells; GC-C activation leads to increased cyclic guanosine monophosphate (cGMP), which, in turn, stimulates secretion of chloride and bicarbonate into the intestinal lumen, mainly by activation of the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel, resulting in increased intestinal fluid and accelerated transit. In animal models, plecanatide has been shown to increase fluid secretion into the gastrointestinal (GI) tract, accelerate intestinal transit, and cause changes in stool consistency. In an animal model of visceral pain, plecanatide reduced abdominal muscle contractions, a measure of intestinal pain. The mechanism has not been studied.
Plecanatide, a guanylate cyclase-C (GC-C) agonist, is a 16-amino acid peptide structurally related to endogenous uroguanylin peptide hormone. The parent drug and its active metabolite bind to GC-C receptors on the luminal surface of the intestinal epithelium, which increases intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP). In turn, increased intracellular concentrations of cGMP trigger a signal-transduction cascade activating the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel. This results in secretion of chloride and bicarbonate into the intestinal lumen causing increased intestinal fluid and accelerated intestinal transit.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
Plecanatide is a recently developed guanylate cyclase-C (GC-C) agonist and the first uroguanylin analog designed to treat chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C). GC-C receptors are found across the length of the intestines and are thought to play a key role in fluid regulation and electrolyte balance. Ligands of the GC-C receptor include endogenous agonists, uroguanylin and guanylin, as well as diarrheagenic, Escherichia coli heat-stable enterotoxins (ST). Plecanatide mimics uroguanylin in its 2 disulfide-bond structure and in its ability to activate GC-Cs in a pH-dependent manner, a feature associated with the presence of acid-sensing residues (Asp2 and Glu3). Linaclotide, a synthetic analog of STh (a 19 amino acid member of ST family), contains the enterotoxin's key structural elements, including the presence of three disulfide bonds. Linaclotide, like STh, activates GC-Cs in a pH-independent manner due to the absence of pH-sensing residues. In this study, molecular dynamics simulations compared the stability of plecanatide and linaclotide to STh. Three-dimensional structures of plecanatide at various protonation states (pH 2.0, 5.0, and 7.0) were simulated with GROMACS software. Deviations from ideal binding conformations were quantified using root mean square deviation values. Simulations of linaclotide revealed a rigid conformer most similar to STh. Plecanatide simulations retained the flexible, pH-dependent structure of uroguanylin. The most active conformers of plecanatide were found at pH 5.0, which is the pH found in the proximal small intestine. GC-C receptor activation in this region would stimulate intraluminal fluid secretion, potentially relieving symptoms associated with CIC and IBS-C.
PMID:28357122 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5368960 Brancale A et al; Pharmacol Res Perspect 5 (2): e00295 (2017)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
28
PharmaCompass offers a list of Plecanatide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Plecanatide manufacturer or Plecanatide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Plecanatide manufacturer or Plecanatide supplier.
PharmaCompass also assists you with knowing the Plecanatide API Price utilized in the formulation of products. Plecanatide API Price is not always fixed or binding as the Plecanatide Price is obtained through a variety of data sources. The Plecanatide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Plecanatide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Plecanatide, including repackagers and relabelers. The FDA regulates Plecanatide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Plecanatide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Plecanatide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Plecanatide supplier is an individual or a company that provides Plecanatide active pharmaceutical ingredient (API) or Plecanatide finished formulations upon request. The Plecanatide suppliers may include Plecanatide API manufacturers, exporters, distributors and traders.
click here to find a list of Plecanatide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Plecanatide DMF (Drug Master File) is a document detailing the whole manufacturing process of Plecanatide active pharmaceutical ingredient (API) in detail. Different forms of Plecanatide DMFs exist exist since differing nations have different regulations, such as Plecanatide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Plecanatide DMF submitted to regulatory agencies in the US is known as a USDMF. Plecanatide USDMF includes data on Plecanatide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Plecanatide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Plecanatide suppliers with USDMF on PharmaCompass.
A Plecanatide written confirmation (Plecanatide WC) is an official document issued by a regulatory agency to a Plecanatide manufacturer, verifying that the manufacturing facility of a Plecanatide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Plecanatide APIs or Plecanatide finished pharmaceutical products to another nation, regulatory agencies frequently require a Plecanatide WC (written confirmation) as part of the regulatory process.
click here to find a list of Plecanatide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Plecanatide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Plecanatide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Plecanatide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Plecanatide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Plecanatide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Plecanatide suppliers with NDC on PharmaCompass.
Plecanatide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Plecanatide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Plecanatide GMP manufacturer or Plecanatide GMP API supplier for your needs.
A Plecanatide CoA (Certificate of Analysis) is a formal document that attests to Plecanatide's compliance with Plecanatide specifications and serves as a tool for batch-level quality control.
Plecanatide CoA mostly includes findings from lab analyses of a specific batch. For each Plecanatide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Plecanatide may be tested according to a variety of international standards, such as European Pharmacopoeia (Plecanatide EP), Plecanatide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Plecanatide USP).

