
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
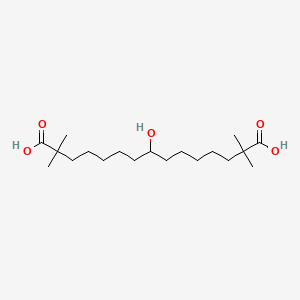

1. 8-hydroxy-2,2,14,14-tetramethylpentadecanedioic Acid
2. Esp-55016
3. Esp55016
4. Etc-1002
5. Nexletol
6. Nilemdo
1. Etc-1002
2. 738606-46-7
3. 8-hydroxy-2,2,14,14-tetramethylpentadecanedioic Acid
4. Nexletol
5. Esp-55016
6. Nilemdo
7. Etc1002
8. Etc 1002
9. Esp 55016
10. 1ej6z6q368
11. Mfcd18800820
12. Etc-1002;esp-55016
13. Pentadecanedioic Acid, 8-hydroxy-2,2,14,14-tetramethyl-
14. Bempedoate
15. Unii-1ej6z6q368
16. Bempedoic Acid [usan:inn]
17. Bempedoic-acid
18. Bempedoic Acid
19. Esp-55016
20. Acido Bempedoico
21. Acide Bempedoique
22. Acidum Bempedoicum
23. Nexletol (tn)
24. Bempedoic Acid (usan/inn)
25. Bempedoic Acid [inn]
26. Bempedoic Acid [jan]
27. Bempedoic Acid [usan]
28. Schembl185768
29. Gtpl8321
30. Bempedoic Acid [who-dd]
31. Chembl3545313
32. Chebi:149601
33. Dtxsid401027952
34. Amy31933
35. Bcp16083
36. Esp55016
37. Ex-a1243
38. Zinc3948738
39. Bempedoic Acid [orange Book]
40. S7953
41. Akos027439916
42. Ccg-267969
43. Cs-3952
44. Db11936
45. Nexlizet Component Bempedoic Acid
46. Ac-29040
47. As-49804
48. Hy-12357
49. Sy244715
50. Bempedoic Acid Component Of Nexlizet
51. Bempedoic Acid(etc-1002;esp-55016)
52. Db-108321
53. D10691
54. N10681
55. A905695
56. Q27075007
57. 8-hydroxy-2,2,14,14-tetramethylpentadecanedioic Acid;etc-1002
| Molecular Weight | 344.5 g/mol |
|---|---|
| Molecular Formula | C19H36O5 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 14 |
| Exact Mass | 344.25627424 g/mol |
| Monoisotopic Mass | 344.25627424 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 351 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Bempedoic acid is indicated as an adjunct to diet and maximally tolerated statin therapy for adults with heterozygous familial hypercholesterolemia or existing atherosclerotic cardiovascular disease that warrants additional lowering of LDL-C. The combination of bempedoic and ezetimibe is also indicated with diet management and maximally tolerated statin therapy to treat elevated LDL-C levels in adults with heterozygous familial hypercholesterolemia or existing atherosclerotic cardiovascular disease who require further lowering of LDL-C.
FDA Label
Nilemdo is indicated in adults with primary hypercholesterolaemia (heterozygous familial and non familial) or mixed dyslipidaemia, as an adjunct to diet:
- in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL C goals with the maximum tolerated dose of a statin (see sections 4. 2, 4. 3, and 4. 4) or,
- alone or in combination with other lipid-lowering therapies in patients who are statin intolerant, or for whom a statin is contraindicated.
Bempedoic acid inhibits the synthesis of cholesterol in the liver, reducing LDL-C levels. This reduces the development of atherosclerotic plaques that may increase the risk of cardiovascular events. Earlier clinical trials studying the effects of bempedoic acid showed a dosedependent reduction of LDLC levels in addition to decreased LDL particle number, and reduced levels of apolipoprotein B, nonHDL cholesterol, and highsensitivity Creactive protein. Due to its unique mechanism of action, bempedoic acid is not associated with myositis, an adverse effect that frequently accompanies statin therapy. More recent trials have supported that this drug significantly decreases LDL-C levels after 12 weeks of therapy and provides additional lowering of LDL-C when combined with ezetimibe and statin therapy. The effects of bempedoic acid on mortality are currently unknown.
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
Hypolipidemic Agents
Substances that lower the levels of certain LIPIDS in the BLOOD. They are used to treat HYPERLIPIDEMIAS. (See all compounds classified as Hypolipidemic Agents.)
C10AX
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AX - Other lipid modifying agents
C10AX15 - Bempedoic acid
Absorption
Bempedoic acid is rapidly absorbed in the small intestine. The Tmax of the 180mg tablet is estimated at 3.5 hours.
Route of Elimination
Bempedoic acid's conjugates are primarily eliminated via the urine (70%) and the feces (30%). A total of 5% of the unchanged drug is excreted in the urine and feces, combined.
Volume of Distribution
The apparent volume of distribution of bempedoic acid is about 18L.
Clearance
The clearance (CL/F) of bempedoic acid at steady state was estimated at 11.2 mL/min during clinical trials.
The two main metabolites of bempedoic metabolism are ETC-1002-CoA and ESP15228. Bempedoic acid is primarily eliminated via the metabolism of its acyl glucuronide. This drug is reversibly converted to an active metabolite (ESP15228) based on observations during in vitro studies. Both compounds resulting from the metabolism of bempedoic acid are metabolized to become inactive glucuronide conjugates by the enzyme UGT2B7.
The half-life of bempedoic acid ranges between 15 and 24 hours. Prescribing information indicates a clearance of 21 hours +/- 11 hours.
Normally, LDL cholesterol is produced in the liver and circulates in the blood. When the blood becomes saturated, excess LDL deposits in blood vessels including the coronary arteries, increasing the risk of cardiovascular events. Bempedoic acid is a prodrug that requires activation in the liver. The very-long-chain acyl-CoA synthetase-1 (ACSVL1) enzyme is responsible for its activation to ETC-1002-CoA, the pharmacologically active metabolite. ATP lyase (also known as ATP synthase) plays an important part of cholesterol synthesis. BETC-1002-CoA directly inhibits this enzyme after the parent drug is activated in the liver by coenzyme A (CoA). This inhibition leads to upregulation of the LDL cholesterol receptor, reducing serum LDL-C via increased uptake and LDL clearance in the liver. By the above mechanisms, bempedoic acid causes a total decrease of circulating LDL-C that normally damages blood vessels and leads to atherosclerosis. Lastly, ETC-1002 activates AMP-activated protein kinase (AMPK) in rodents, which inhibits the synthesis of cholesterol via the inhibition of HMG-CoA reductase. The relevance of this to humans is unknown.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Tablet
Dosage Strength : 180MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 180MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Regulatory Info : Lead Market Dossiers- Under De...
Registration Country : India
Brand Name :
Dosage Form : Oral Solid Dosage Form
Dosage Strength : 120MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Lead Market Dossiers- Under De...
Registration Country : India
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Packaging :
Regulatory Info : Lead Market Dossiers- Under De...
Dosage : Oral Solid Dosage Form
Dosage Strength : 120MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Regulatory Info : Lead Market Dossiers- Under De...
Registration Country : India
Brand Name :
Dosage Form : Oral Solid Dosage Form
Dosage Strength : 240MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Lead Market Dossiers- Under De...
Registration Country : India
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Packaging :
Regulatory Info : Lead Market Dossiers- Under De...
Dosage : Oral Solid Dosage Form
Dosage Strength : 240MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Regulatory Info : Lead Market Dossiers- Under De...
Registration Country : India
Brand Name :
Dosage Form : Oral Solid Dosage Form
Dosage Strength : 180MG; 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Lead Market Dossiers- Under De...
Registration Country : India
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Packaging :
Regulatory Info : Lead Market Dossiers- Under De...
Dosage : Oral Solid Dosage Form
Dosage Strength : 180MG; 10MG
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Regulatory Info :
Registration Country : Hungary
Brand Name : Bempedoic acid
Dosage Form : Film-Coated Tablet
Dosage Strength : 180MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Packaging :
Regulatory Info :
Dosage : Film-Coated Tablet
Dosage Strength : 180MG
Brand Name : Bempedoic acid
Approval Date :
Application Number :
Registration Country : Hungary
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : Bempalip
Dosage Form : Tablet
Dosage Strength : 180MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 180MG
Brand Name : Bempalip
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Egypt
Brand Name : Zetacolest
Dosage Form : Film-Coated Tablet
Dosage Strength : 180MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Egypt

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Film-Coated Tablet
Dosage Strength : 180MG
Brand Name : Zetacolest
Approval Date :
Application Number :
Registration Country : Egypt

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
20
PharmaCompass offers a list of Bempedoic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Bempedoic Acid manufacturer or Bempedoic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Bempedoic Acid manufacturer or Bempedoic Acid supplier.
PharmaCompass also assists you with knowing the Bempedoic Acid API Price utilized in the formulation of products. Bempedoic Acid API Price is not always fixed or binding as the Bempedoic Acid Price is obtained through a variety of data sources. The Bempedoic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl-, including repackagers and relabelers. The FDA regulates Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- supplier is an individual or a company that provides Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- active pharmaceutical ingredient (API) or Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- finished formulations upon request. The Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- suppliers may include Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- API manufacturers, exporters, distributors and traders.
click here to find a list of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- DMF (Drug Master File) is a document detailing the whole manufacturing process of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- active pharmaceutical ingredient (API) in detail. Different forms of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- DMFs exist exist since differing nations have different regulations, such as Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- DMF submitted to regulatory agencies in the US is known as a USDMF. Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- USDMF includes data on Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl-'s chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- suppliers with USDMF on PharmaCompass.
A Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- written confirmation (Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- WC) is an official document issued by a regulatory agency to a Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- manufacturer, verifying that the manufacturing facility of a Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- APIs or Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- finished pharmaceutical products to another nation, regulatory agencies frequently require a Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- WC (written confirmation) as part of the regulatory process.
click here to find a list of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- suppliers with NDC on PharmaCompass.
Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- GMP manufacturer or Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- GMP API supplier for your needs.
A Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- CoA (Certificate of Analysis) is a formal document that attests to Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl-'s compliance with Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- specifications and serves as a tool for batch-level quality control.
Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- CoA mostly includes findings from lab analyses of a specific batch. For each Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- may be tested according to a variety of international standards, such as European Pharmacopoeia (Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- EP), Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pentadecanedioic acid, 8-hydroxy-2,2,14,14-tetramethyl- USP).
