
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
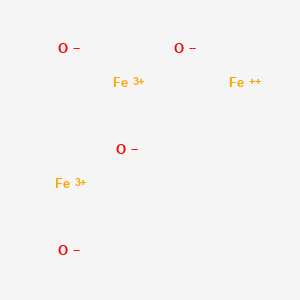
1. Ami 25
2. Ami25
3. Aquamag100
4. Carboxydextran-coated Superparamagnetic Iron Oxide
5. Dextran-coated Spio
6. Dextran-coated Superparamagnetic Iron Oxide
7. Endorem
8. Feridex
9. Ferucarbotran
10. Ferumoxides Non-stoichiometric Magnetite
11. Resovist
12. Sh U 555a
13. Shu 555a
14. Shu-555a
15. Win 39996
1. Ami-25
2. 119683-68-0
3. G6n3j05w84
4. Iron(2+);iron(3+);oxygen(2-)
5. Ferumoxytol Non-stoichiometric Magnetite
6. Feridex
7. Feridex I.v.
8. Endorem
9. Ferumoxides [usan]
10. Superparamagnetic Iron Oxide
11. Unii-g6n3j05w84
12. Ferumoxides [usan:usp:ban]
13. Ccris 6722
14. Black Iron Oxide
15. Hsdb 8072
16. Ferroso Ferric Oxide
17. Cishi
18. Magnetitum
19. Ferumoxides Non-stoichiometric Magnetite
20. Magnetic Stone
21. Black Rouge
22. Ci Shi
23. Ferrosferric Oxide
24. Ferumoxides [mi]
25. Magnetitum [chp]
26. Ferric Oxide Black
27. Ferumoxides [jan]
28. Ferumoxides [usp]
29. Black Magnetic Oxide
30. Ferumoxides [vandf]
31. Ferumoxides [mart.]
32. Bayoxide E 8713h
33. Ferumoxides [who-dd]
34. Tarox Black Bl 100p
35. Clh5ft6412
36. Ferrosoferric Oxide [ii]
37. Ferrosoferric Oxide [mi]
38. Ferrosoferric Oxide [nf]
39. Ins No.172(i)
40. Ferumoxides [orange Book]
41. Ci 77499 [inci]
42. Ins-172(i)
43. Ferrosoferric Oxide [who-dd]
44. Db06215
45. Ci 77499
46. E-172(i)
47. C.i. 77499
48. Ci (1975) No. 77499
49. Iron Oxide Crystal Is Inverse Spinel (x-ray Data)
50. Fe(ii) And Fe(iii) Are Present (mossbauer Spectroscopy
51. Physical Form Is A Colloidal Particle Of Nonstoichiometric Magnetite
| Molecular Weight | 231.53 g/mol |
|---|---|
| Molecular Formula | Fe3O4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 231.784465 g/mol |
| Monoisotopic Mass | 231.784465 g/mol |
| Topological Polar Surface Area | 4 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 7 |
Contrast Media; Magnetite Nanoparticles
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Feridex I.V. is indicated for I.V. administration as an adjunct to MRI (in adult patients) to enhance the T2 weighted images used in the detection and evaluation of lesions of the liver that are associated with an alteration in the reticuloendothelial system (RES). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
... There remains virtually no evidence of the fate of stem cells in these human studies, primarily owing to safety concerns associated with the use of cell-labeling strategies. This study, utilized two cell types that are used extensively in cardiac regeneration studies, namely bone marrow-derived human mononuclear cells and C2C12 skeletal myoblasts. The US FDA-approved compounds feridex (ferumoxide) and protamine sulfate (as a transfection agent) were used in combination for cellular labeling. /Investigators/ assessed the effect of this cell labeling strategy on cellular viability, proliferation and differentiation both in vitro and in vivo. The ferumoxide-protamine sulfate combination had no effect on cellular viability, proliferation or differentiation. /Investigators/ show that the labeled human mononuclear cells were easily identified within the rat myocardium 1 month following injection into the myocardium. These human cells expressed human-specific cardiac troponin I, whereas the neighboring rat myocardium did not. Furthermore, /investigators/ demonstrated that this labeling strategy can be used with high accuracy for magnetic separation of the labeled cells based on the intracellular ferumoxide particles. The ferumoxide-protamine sulfate combination can be used safely and effectively to enhance the detection and isolation of cardiogenic stem cell populations.
PMID:18947305 Sadek H et al; Regen Med 3 (6): 807-16 (2008)
Feridex i.v. is contraindicated in patients with known allergic or hypersensitivity reactions to parenteral iron, parenteral dextran, parenteral iron-dextran, or parenteral iron-polysaccharide preparations.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Anaphylactic-like reactions and hypotension have been noted in some patients receiving feridex i.v., other iron and dextran containing formulations, or radiographic contrast media. In clinical trials, anaphylactic and allergic adverse events occurred in 11/2240 (0.5%) of the patients who received feridex i.v. These events include dyspnea, other respiratory symptoms, angioedema, generalized urticaria, and hypotension; and required treatment.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Acute severe back, leg or groin pain occurred in some patients. In clinical trials, 55/2240 (2.5%) of the patients experienced pain that was severe enough to cause interruption or discontinuation of the infusion. In most patients, the symptoms developed within 1 to 15 minutes (up to 45 minutes). Some patients required treatment with corticosteroids, intravenous fluids or muscle relaxants. Pain may occur alone or with other symptoms such as hypotension and dyspnea. Patients with both pain and allergic symptoms received treatment with a combination of medications directed toward each event.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Patients with autoimmune disease have not been studied with feridex i.v., but have been reported in published literature to have a high rate of adverse reactions to injectable iron formulations.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
For more Drug Warnings (Complete) data for Ferumoxides (17 total), please visit the HSDB record page.
This drug is indicated for the treatment of iron deficiency anemia in adult patients who have experienced intolerance to oral iron or have experienced an unsatisfactory response to oral iron or who have chronic kidney disease (CKD).
FDA Label
The pharmacodynamic effect of ferumoxytol on hematologic indexes such as Hgb (hemoglobin), serum ferritin, and TSAT (transferrin saturation) were studied and measured as primary and secondary endpoints in clinical efficacy studies. Feraheme (ferumoxytol) reached the primary endpoint with statistical significance (p<0.001) in all three trials versus oral iron. Ferumoxytol has been examined as a contrast agent for magnetic resonance imaging (MRI) studies. Because ferumoxytol is a very small superparamagnetic iron oxide (USPIO) with a polysaccharide coating, it may be administered via the intravenous bolus route without mast cell degranulation, which is an attributable property for magnetic resonance angiography and perfusion imaging. Unlike gadolinium, ferumoxytol crosses the blood-brain barrier at a slow pace and is considered a 'blood pool' agent. Ferumoxytol stays in the intravascular space and offers a longer time period for data acquisition during an MRI study so that data can be repeatedly obtained over a period of several minutes to hours with only small losses of intravascular signal intensity and minimal soft tissue enhancement. Iron-containing proteins and enzymes are important in oxidation-reduction reactions, particularly those in the mitochondria. Iron is a part of myoglobin and several heme-enzymes, including the cytochromes, _catalase_, and _peroxidase_. Iron is an essential component of the m_etalloflavoprotein_ enzymes and the mitochondrial enzyme _alpha-glycerophosphate oxidase_. In addition, iron is a cofactor for enzymes such as _aconitase_ and _tryptophan pyrrolase_. Iron deficiency cause anemia and decreased oxygen delivery. This also reduces the metabolism of muscle and decreases mitochondrial activity. Iron deficiency may also cause defects in both learning or thermoregulation. Therefore, iron is important to several metabolic functions in addition to erythropoiesis.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
Absorption
Bioavailability studies were not conducted as ferumoxytol has been developed for IV administration only. Iron therapy dosage is individualized according to specific goals for blood iron concentrations, iron storage parameters (e.g., ferritin, transferrin saturation), and serum hemoglobin concentrations. Iron toxicity is possible with excessive or unnecessary iron therapy. Systemic iron is stored in ferritin and hemosiderin, which are utilized for future production of hemoglobin. The absorption of iron depends on the route of administration. The tissue that first clears parenterally ingested iron from the plasma determines its bioavailability. If the reticuloendothelial system clears iron effectively, only small amounts will become available over time to the bone marrow. Transferrin accepts iron from the intestinal tract and also from sites of hemoglobin storage and destruction.
Route of Elimination
Iron can either become a component of intracellular ferritin or be transferred to erythroid precursor cells.
Volume of Distribution
The population mean estimates for volume of distribution of the central compartment (V(1)), maximum elimination rate (V(max)), and ferumoxytol concentration at which rate of metabolism would be one-half of V(max) (K(m)) were 2.71 l, 14.3 mg/hr, and 77.5 mg/L, respectively.
Clearance
Since there is no renal clearance, ferumoxytol is safe in renal failure patients. One study estimated the clearance to be **0.0221 L/h**.
Three healthy, adult male volunteers received a dose of Feridex I.V. 0.56 mg of Fe/kg (diluted in 100 mL of 5% dextrose and intravenously infused over 30 minutes). In these subjects, the mean +/- SD peak serum iron concentration was 5.5 +/- 0.6 ug/mL and total clearance 28.5 +/- 1.6 mL/min.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Feridex i.v. was completely cleared from the blood by 25 hours after administration. Less than 2% of the drug was excreted in the urine, as expected for iron.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
It is not known whether Feridex i.v. is excreted in human milk.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
At 24 hours, serum iron increased and the percent saturation of iron binding capacity decreased in a dose-dependent fashion. By 7 days, serum iron returned to pre-administration levels, and serum ferritin increased. These results are consistent with the iron in Feridex i.v. entering the usual iron metabolism cycle. Animal pharmacokinetics studies were consistent with these results in humans.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Imaging studies in rats showed a large decrease in liver signal intensity for the first 24 hours after dosing, followed by a gradual return to normal over 7 days. Radiotracer studies in rats were consistent with the iron in Feridex i.v. becoming part of the body iron pool. Histological studies in rats showed that the iron was in the RES and that it disappeared from the RES over 7 to 14 days with all evidence of iron gone by 14-28 days.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Ferumoxytol metabolism is not dependent on renal function. It is removed from the circulation by the reticuloendothelial system of the liver, spleen, and bone marrow. Iron, bound to transferrin, is then transported in the plasma and distributed to the bone marrow for the synthesis of hemoglobin, to the reticuloendothelial system for storage, and to all cells for enzymes containing iron, and to placental cells if needed to meet fetal needs. Transferrin eventually becomes available for recycling. In normal adults, 90% of metabolized iron is conserved and reutilized repeatedly.
The iron in Feridex i.v. enters the normal body iron metabolism cycle as evidenced by transient increases in serum iron values one day after administration and increase in serum ferritin values 7 days after administration. The amount of iron contained in a single dose is 39 mg for a 70 kg individual. This is less than 1/5 the amount of iron contained in one unit of whole blood.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
The pharmacokinetic (PK) behavior of Feraheme has been studied in healthy subjects and in patients with stage 5D of chronic kidney disease, on hemodialysis. Feraheme showed dose-dependent, capacity-limited elimination from the plasma with a half-life of **approximately 15 hours*
in humans.
... Elimination half-life was 2.4 +/- 0.2 hours and total clearance 28.5 +/- 1.6 mL/min.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Feraheme (ferumoxytol) is comprised of a superparamagnetic iron oxide that is coated with a carbohydrate shell, aiding in the isolation the bioactive iron from plasma components until the iron-carbohydrate complex enters the reticuloendothelial system macrophages of the liver, spleen and the bone marrow. The iron is then released from the iron-carbohydrate complex within vesicles located in the macrophages. Iron then either enters the intracellular storage of iron (e.g., ferritin) or can be transferred to plasma transferrin for its transport to erythroid precursor cells for incorporation into hemoglobin. A therapeutic response to iron therapy depends upon the individual's iron stores and ability to utilize the iron. The systemic use of iron is influenced by the cause of the deficiency in addition to the illnesses/conditions that may affect erythropoiesis. Iron therapy by itself does not increase red blood cell (RBC) production. Administration of iron improves only the anemia associated with iron deficiency. Iron-containing proteins and enzymes are essential in oxidation-reduction reactions, particularly those in the mitochondria. Iron is a part of myoglobin and various heme-enzymes, including the cytochromes, catalase, and peroxidase. Iron is an important component of the _metalloflavoprotein _enzymes as well as the mitochondrial enzyme _alpha-glycerophosphate oxidase_. In addition, iron serves as a cofactor for enzymes such as _aconitase _and tryptophan _pyrrolase_. Iron deficiency leads anemia and decreased oxygen delivery, but also reduces muscle metabolism and decreases mitochondrial activity. Iron deficiency may also lead to defects in both learning and body thermoregulation. Therefore, iron is imperative to several metabolic functions in addition to erythropoiesis. After intravenous administration, ferumoxytol replaces iron stores with less frequent side effects compared to the use of oral iron therapy. In addition, this agent generates T1 relaxation, producing a magnetic field and enhancing T2 relaxation, thereby darkening contrast media-containing structures in magnetic resonance imaging (MRI). Due to small particle size, ferumoxytol remains in the intravascular space for a prolonged period and so may be used as a blood pool agent. T1 and T2, in radiology, refer to the timing of radiofrequency pulse sequences used to make images. The timing used to create T1 images results in images which emphasize fat tissue. The timing of radiofrequency pulse sequences utilized to create T2 images results in images which emphasize fat AND water within the body.
Feridex i.v. is an intravenously injected colloidal superparamagnetic iron oxide associated with dextran. It is a magnetic resonance imaging (MRI) contrast agent and is taken up by cells of the reticuloendothelial system (RES).
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Feridex i.v. shortens the relaxation times for nearby hydrogen atoms and reduces signal intensity in normal tissues. This results in signal loss (image darkening ) on mid T1/T2 or strongly T2-weighted images. Tissues with decreased RES function (e.g., metastases, primary liver cancer, cysts and various benign tumors, adenomas, and hyperplasia) retain their native signal intensity, so the contrast between normal and abnormal tissue is increased.
US Natl Inst Health; DailyMed. Current Medication Information for feridex (ferumoxides) solution (March 2008). Available from, as of July 9, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=82ceb312-3aaa-4a5c-9849-7f246cc06ded
Magnetic resonance imaging (MRI) maps information about tissues spatially and functionally. Protons (hydrogen nuclei) are widely used to create images because of their abundance in water molecules. Water comprises about 80% of most soft tissues. The contrast of proton MRI depends mainly on the density of nuclear (proton spins), the relaxation times of the nuclear magnetization (T1, longitudinal and T2, transverse), the magnetic environment of the tissues, and the blood flow to the tissues. However, insufficient contrast between normal and diseased tissues requires the development of contrast agents. Most of the contrast agents affect the T1 and T2 relaxation of the surrounding nuclei, mainly the protons of water. T-2* is the spin-spin relaxation time composed of variations from molecular interactions and intrinsic magnetic heterogeneities of tissues in the magnetic field (1). Superparamagnetic iron oxide (SPIO) structure is composed of ferric iron (Fe3+) and ferrous iron (Fe2+) in the general formula of Fe2 (3+)OFe(2+)O. The iron oxides particles are coated with a layer of dextran or other polysaccharide. These particles have a large combined magnetic moments or spins which are randomly rotated in the absence of an applied magnetic field. SPIO is used mainly as a T2 contrast agent in MRI though it can shorten both T1 and T2/T2* relaxation processes. SPIO particle uptake into the reticuloendothelial system (RES) is by endocytosis or phagocytosis. SPIO particles are taken up by phagocytic cells such as monocytes, macrophages, and oligodendroglial cells. A variety of cells can also be labeled with these particles for cell trafficking and tumor-specific imaging studies. SPIO agents are classified by their sizes with coating material (about 20 nm to 3,500 nm in diameters) as large SPIO agents (Ferumoxsil or AMI-121, Ferucarbotran, OMP), standard SPIO (SSPIO) agents (Ferumoxides or AMI-25, SHU 555A), ultrasmall SPIO (USPIO) agents (Ferumoxtran or AMI-277, NC100150) and monocrystalline iron oxide nanoparticles (MION) agents. Ferumoxides are composed of iron particles of about 5 nm, and the hydrodynamic diameter is about 80-150 nm. The crystals are covered with a layer of dextran. Ferumoxides are classified as SSPIO. Ferumoxides have been tested in clinical trials as negative contrast agents that decrease signal on T2 images. Ferumoxides have been used in liver, spleen, and myocardial perfusion MR imaging.[Leung K; Ferumoxides. 2004 Nov 1 (Updated 2007 Dec 12). In: Molecular Imaging and Contrast Agent Database (MICAD)
Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2011. Available from, as of July 9, 2012: https://www.ncbi.nlm.nih.gov/books/NBK23037/
ABOUT THIS PAGE
21
PharmaCompass offers a list of Ferumoxides API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ferumoxides manufacturer or Ferumoxides supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ferumoxides manufacturer or Ferumoxides supplier.
PharmaCompass also assists you with knowing the Ferumoxides API Price utilized in the formulation of products. Ferumoxides API Price is not always fixed or binding as the Ferumoxides Price is obtained through a variety of data sources. The Ferumoxides Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ferumoxides manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ferumoxides, including repackagers and relabelers. The FDA regulates Ferumoxides manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ferumoxides API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Ferumoxides supplier is an individual or a company that provides Ferumoxides active pharmaceutical ingredient (API) or Ferumoxides finished formulations upon request. The Ferumoxides suppliers may include Ferumoxides API manufacturers, exporters, distributors and traders.
click here to find a list of Ferumoxides suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ferumoxides DMF (Drug Master File) is a document detailing the whole manufacturing process of Ferumoxides active pharmaceutical ingredient (API) in detail. Different forms of Ferumoxides DMFs exist exist since differing nations have different regulations, such as Ferumoxides USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ferumoxides DMF submitted to regulatory agencies in the US is known as a USDMF. Ferumoxides USDMF includes data on Ferumoxides's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ferumoxides USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ferumoxides suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ferumoxides as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ferumoxides API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ferumoxides as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ferumoxides and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ferumoxides NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ferumoxides suppliers with NDC on PharmaCompass.
Ferumoxides Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ferumoxides GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ferumoxides GMP manufacturer or Ferumoxides GMP API supplier for your needs.
A Ferumoxides CoA (Certificate of Analysis) is a formal document that attests to Ferumoxides's compliance with Ferumoxides specifications and serves as a tool for batch-level quality control.
Ferumoxides CoA mostly includes findings from lab analyses of a specific batch. For each Ferumoxides CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ferumoxides may be tested according to a variety of international standards, such as European Pharmacopoeia (Ferumoxides EP), Ferumoxides JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ferumoxides USP).

