
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
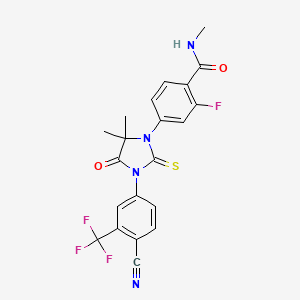

1. 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl)-2-fluoro-n-(methyl-d3)benzamide
2. 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl)-2-fluoro-n-methyl-benzamide
3. Enzalutamide D3
4. Hc 1119
5. Hc-1119
6. Mdv 3100
7. Mdv-3100
8. Mdv3100
9. Xtandi
1. 915087-33-1
2. Mdv3100
3. Mdv-3100
4. 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-n-methylbenzamide
5. Mdv 3100
6. Enzalutamide (mdv3100)
7. Xtandi
8. Mdv3100 (enzalutamide)
9. 93t0t9gknu
10. Chebi:68534
11. 4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl]-2-fluoro-n-methylbenzamide
12. 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl}-2-fluoro-n-methylbenzamide
13. 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl}-2-fluoro-n-methylbenzamide
14. Benzamide, 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl)-2-fluoro-n-methyl-
15. Enzalutamide [usan]
16. Enzalutamide [usan:inn]
17. Unii-93t0t9gknu
18. Xtandi (tn)
19. Enzalutamide [mi]
20. Enzalutamide; Mdv3100
21. Enzalutamide (jan/usan)
22. Enzalutamide [inn]
23. Enzalutamide [jan]
24. Mdv3100, Aldrichcpr
25. Enzalutamide [vandf]
26. Mls006010067
27. Enzalutamide [who-dd]
28. Schembl189749
29. Gtpl6812
30. Chembl1082407
31. Dtxsid10912307
32. Ex-a046
33. Bcpp000169
34. Enzalutamide [orange Book]
35. Hms3654l07
36. Hms3672m13
37. Hms3744c19
38. Nc-54
39. Amy10296
40. Asp-9785
41. Bcp02361
42. Bbl102957
43. Bdbm50425732
44. Mfcd14155804
45. Nsc755605
46. Nsc766085
47. S1250
48. Stl556766
49. Zinc34806477
50. Akos015851022
51. Mdv-3100;enzalutamide;mdv 3100
52. Bcp9000901
53. Ccg-264879
54. Cs-0317
55. Db08899
56. Nsc-755605
57. Nsc-766085
58. Sb20413
59. Ncgc00263120-01
60. 4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-imidazolidin-1-yl]-2-fluoro-n-methyl-benzamide
61. Ac-26924
62. As-17047
63. Benzamide,4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-n-methyl-
64. Hy-70002
65. Mdv3100, 95%
66. Smr004701227
67. Ft-0670957
68. Sw219288-1
69. A25302
70. D10218
71. Ab01565849_02
72. Sr-01000941580
73. J-519668
74. Q1996756
75. Sr-01000941580-1
76. Brd-k56851771-001-01-9
| Molecular Weight | 464.4 g/mol |
|---|---|
| Molecular Formula | C21H16F4N4O2S |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Exact Mass | 464.09300959 g/mol |
| Monoisotopic Mass | 464.09300959 g/mol |
| Topological Polar Surface Area | 109 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 839 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Xtandi |
| PubMed Health | Enzalutamide (By mouth) |
| Drug Classes | Antiandrogen, Antineoplastic Agent |
| Drug Label | Enzalutamide is an androgen receptor inhibitor. The chemical name is 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl}-2-fluoro-N-methylbenzamide. The molecular weight is 464.44 and molecular formula is C21... |
| Active Ingredient | Enzalutamide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 40mg |
| Market Status | Prescription |
| Company | Astellas |
| 2 of 2 | |
|---|---|
| Drug Name | Xtandi |
| PubMed Health | Enzalutamide (By mouth) |
| Drug Classes | Antiandrogen, Antineoplastic Agent |
| Drug Label | Enzalutamide is an androgen receptor inhibitor. The chemical name is 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl}-2-fluoro-N-methylbenzamide. The molecular weight is 464.44 and molecular formula is C21... |
| Active Ingredient | Enzalutamide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 40mg |
| Market Status | Prescription |
| Company | Astellas |
Enzalutamide is indicated for the treatment of patients with metastatic castration-resistant prostate cancer who have previously received docetaxel.
FDA Label
Xtandi is indicated for:
- the treatment of adult men with metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy (see section 5. 1).
- the treatment of adult men with high-risk non-metastatic castration-resistant prostate cancer (CRPC) (see section 5. 1).
- the treatment of adult men with metastatic CRPC who are asymptomatic or mildly symptomatic after failure of androgen deprivation therapy in whom chemotherapy is not yet clinically indicated (see section 5. 1).
- the treatment of adult men with metastatic CRPC whose disease has progressed on or after docetaxel therapy.
Resitance to enzalutamide therapy has been observed. This may occurred due to an upregulation of NF-B2/p52.
L02BB04
L02BB04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BB - Anti-androgens
L02BB04 - Enzalutamide
Absorption
The pharmacokinetic profile of enzalutamide and N-desmethyl enzalutamide (its major active metabolite) is described by a linear two-compartment model with first-order absorption. Enzalutamide also accumulates. Food does not affect its absorption. Tmax, prostate cancer patients = 1 hour (range of 0.5-3 hours); Cmax, steady state, enzalutamide = 16.6 g/mL; Cmax, steady state, N-desmethyl enzalutamide = 12.7 g/mL; Time to steady state, daily dosing = 28 days;
Route of Elimination
Enzalutamide is primarily eliminated by hepatic metabolism. 71% of the dose is recovered in urine (including only trace amounts of enzalutamide and N-desmethyl enzalutamide), and 14% is recovered in feces (0.4% of dose as unchanged enzalutamide and 1% as N-desmethyl enzalutamide).
Volume of Distribution
Apparent volume of distribution (Vd/F), single oral dose = 110 L
Clearance
Apparent clearance (CL/F), single oral dose = 0.56 L/h (range of 0.33 - 1.02 L/h)
Enzalutamide is hepatically metabolized, primarily by CYP2C8 and CYP3A4. The enzyme that converts enzalutamide to its active metabolite, N-desmethyl enzalutamide, is CYP2C8. The activity of N-desmethyl-enzalutamide is similar to that of the parent compound.
The mean terminal half-life (t1/2) for enzalutamide in patients after a single oral dose is 5.8 days (range 2.8 to 10.2 days). Following a single 160 mg oral dose of enzalutamide in healthy volunteers, the mean terminal t1/2 for N-desmethyl enzalutamide is approximately 7.8 to 8.6 days.
Enzalutamide is a competitive androgen receptor inhibitor that effects multiple stages of the signalling pathway. It is able to inhibit androgen binding to its receptor, androgen receptor nuclear translocation, and subsequent interaction with DNA. As a result, proliferation of prostate cancer cells decreases which ultimately leads to apoptosis and decreased tumour volume.
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 39560
Submission : 2024-04-04
Status : Active
Type : II
 Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35618
Submission : 2021-04-28
Status : Active
Type : II
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
GDUFA
DMF Review : Complete
Rev. Date : 2015-06-11
Pay. Date : 2014-12-30
DMF Number : 29117
Submission : 2015-03-31
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2016-07-06
Pay. Date : 2016-05-13
DMF Number : 29872
Submission : 2016-05-24
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25814
Submission : 2012-02-22
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-05-24
Pay. Date : 2016-12-20
DMF Number : 31196
Submission : 2016-12-30
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2020-06-01
Pay. Date : 2020-05-05
DMF Number : 30279
Submission : 2016-02-29
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 30644
Submission : 2016-07-09
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2016-04-13
Pay. Date : 2016-03-08
DMF Number : 30260
Submission : 2016-03-17
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 30010
Submission : 2015-11-28
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
About the Company : Polpharma API, part of a leading Polish pharmaceutical group, has over 70 years of experience in process development and cGMP manufacturing. We offer end-to-end solutions, from API...
 Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
Aarti Pharmalabs is a partner of choice for APIs & Intermediates and the largest Indian manufacturer of Xanthine Derivatives.
About the Company : Aarti Pharmalab, earlier the pharma division of Aarti Industries, is a leading Indian manufacturer of APIs. It has dedicated facilities to manufacture HPAPIs, corticosteroids, cyto...
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
About the Company : Founded in 1984, DRL is well-known for its generic APIs and its track record in drug product development. It is one of the earliest pharma API manufacturers with a diverse portfoli...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
About the Company : Supriya Lifescience Ltd. specializes in API manufacturing, focusing on therapeutic segments like antihistamines, anti-allergic drugs, vitamins, anaesthetics and anti-asthmatics. Su...
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
About the Company : Founded in 1935, TAPI Technology & API Services has a long-standing tradition of advancing health through innovation and dedication. Today, we proudly build upon this legacy, drivi...
About the Company : In the dinamic pharmaceutical field, DEAFARMA is the reference point for primaries Pharmaceutical Laboratories for over twenty years, even in the national and international territo...

About the Company : Hetero is a research based global pharmaceutical company focused on development, manufacturing and marketing of Active Pharmaceutical Ingredients (APIs), Intermediate Chemicals & F...

About the Company : Established in the year 2004, Sakar is engaged in manufacturing of Pharmaceutical products providing Liquid Orals, Cephalosporin Tablet, Capsule, Dry Powder Syrup, Dry Powder Injec...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
43
PharmaCompass offers a list of Enzalutamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Enzalutamide manufacturer or Enzalutamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Enzalutamide manufacturer or Enzalutamide supplier.
PharmaCompass also assists you with knowing the Enzalutamide API Price utilized in the formulation of products. Enzalutamide API Price is not always fixed or binding as the Enzalutamide Price is obtained through a variety of data sources. The Enzalutamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Enzalutamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Enzalutamide, including repackagers and relabelers. The FDA regulates Enzalutamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Enzalutamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Enzalutamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Enzalutamide supplier is an individual or a company that provides Enzalutamide active pharmaceutical ingredient (API) or Enzalutamide finished formulations upon request. The Enzalutamide suppliers may include Enzalutamide API manufacturers, exporters, distributors and traders.
click here to find a list of Enzalutamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Enzalutamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Enzalutamide active pharmaceutical ingredient (API) in detail. Different forms of Enzalutamide DMFs exist exist since differing nations have different regulations, such as Enzalutamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Enzalutamide DMF submitted to regulatory agencies in the US is known as a USDMF. Enzalutamide USDMF includes data on Enzalutamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Enzalutamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Enzalutamide suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Enzalutamide Drug Master File in Korea (Enzalutamide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Enzalutamide. The MFDS reviews the Enzalutamide KDMF as part of the drug registration process and uses the information provided in the Enzalutamide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Enzalutamide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Enzalutamide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Enzalutamide suppliers with KDMF on PharmaCompass.
A Enzalutamide written confirmation (Enzalutamide WC) is an official document issued by a regulatory agency to a Enzalutamide manufacturer, verifying that the manufacturing facility of a Enzalutamide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Enzalutamide APIs or Enzalutamide finished pharmaceutical products to another nation, regulatory agencies frequently require a Enzalutamide WC (written confirmation) as part of the regulatory process.
click here to find a list of Enzalutamide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Enzalutamide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Enzalutamide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Enzalutamide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Enzalutamide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Enzalutamide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Enzalutamide suppliers with NDC on PharmaCompass.
Enzalutamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Enzalutamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Enzalutamide GMP manufacturer or Enzalutamide GMP API supplier for your needs.
A Enzalutamide CoA (Certificate of Analysis) is a formal document that attests to Enzalutamide's compliance with Enzalutamide specifications and serves as a tool for batch-level quality control.
Enzalutamide CoA mostly includes findings from lab analyses of a specific batch. For each Enzalutamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Enzalutamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Enzalutamide EP), Enzalutamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Enzalutamide USP).
