

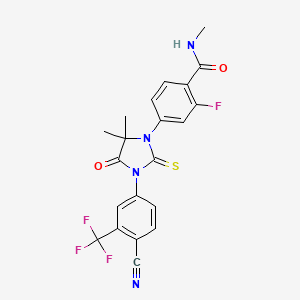

1. 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl)-2-fluoro-n-(methyl-d3)benzamide
2. 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl)-2-fluoro-n-methyl-benzamide
3. Enzalutamide D3
4. Hc 1119
5. Hc-1119
6. Mdv 3100
7. Mdv-3100
8. Mdv3100
9. Xtandi
1. 915087-33-1
2. Mdv3100
3. Mdv-3100
4. 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-n-methylbenzamide
5. Mdv 3100
6. Enzalutamide (mdv3100)
7. Xtandi
8. Mdv3100 (enzalutamide)
9. 93t0t9gknu
10. Chebi:68534
11. 4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl]-2-fluoro-n-methylbenzamide
12. 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl}-2-fluoro-n-methylbenzamide
13. 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl}-2-fluoro-n-methylbenzamide
14. Benzamide, 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl)-2-fluoro-n-methyl-
15. Enzalutamide [usan]
16. Enzalutamide [usan:inn]
17. Unii-93t0t9gknu
18. Xtandi (tn)
19. Enzalutamide [mi]
20. Enzalutamide; Mdv3100
21. Enzalutamide (jan/usan)
22. Enzalutamide [inn]
23. Enzalutamide [jan]
24. Mdv3100, Aldrichcpr
25. Enzalutamide [vandf]
26. Mls006010067
27. Enzalutamide [who-dd]
28. Schembl189749
29. Gtpl6812
30. Chembl1082407
31. Dtxsid10912307
32. Ex-a046
33. Bcpp000169
34. Enzalutamide [orange Book]
35. Hms3654l07
36. Hms3672m13
37. Hms3744c19
38. Nc-54
39. Amy10296
40. Asp-9785
41. Bcp02361
42. Bbl102957
43. Bdbm50425732
44. Mfcd14155804
45. Nsc755605
46. Nsc766085
47. S1250
48. Stl556766
49. Zinc34806477
50. Akos015851022
51. Mdv-3100;enzalutamide;mdv 3100
52. Bcp9000901
53. Ccg-264879
54. Cs-0317
55. Db08899
56. Nsc-755605
57. Nsc-766085
58. Sb20413
59. Ncgc00263120-01
60. 4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-imidazolidin-1-yl]-2-fluoro-n-methyl-benzamide
61. Ac-26924
62. As-17047
63. Benzamide,4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-n-methyl-
64. Hy-70002
65. Mdv3100, 95%
66. Smr004701227
67. Ft-0670957
68. Sw219288-1
69. A25302
70. D10218
71. Ab01565849_02
72. Sr-01000941580
73. J-519668
74. Q1996756
75. Sr-01000941580-1
76. Brd-k56851771-001-01-9
| Molecular Weight | 464.4 g/mol |
|---|---|
| Molecular Formula | C21H16F4N4O2S |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Exact Mass | 464.09300959 g/mol |
| Monoisotopic Mass | 464.09300959 g/mol |
| Topological Polar Surface Area | 109 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 839 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Xtandi |
| PubMed Health | Enzalutamide (By mouth) |
| Drug Classes | Antiandrogen, Antineoplastic Agent |
| Drug Label | Enzalutamide is an androgen receptor inhibitor. The chemical name is 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl}-2-fluoro-N-methylbenzamide. The molecular weight is 464.44 and molecular formula is C21... |
| Active Ingredient | Enzalutamide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 40mg |
| Market Status | Prescription |
| Company | Astellas |
| 2 of 2 | |
|---|---|
| Drug Name | Xtandi |
| PubMed Health | Enzalutamide (By mouth) |
| Drug Classes | Antiandrogen, Antineoplastic Agent |
| Drug Label | Enzalutamide is an androgen receptor inhibitor. The chemical name is 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl}-2-fluoro-N-methylbenzamide. The molecular weight is 464.44 and molecular formula is C21... |
| Active Ingredient | Enzalutamide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 40mg |
| Market Status | Prescription |
| Company | Astellas |
Enzalutamide is indicated for the treatment of patients with metastatic castration-resistant prostate cancer who have previously received docetaxel.
FDA Label
Xtandi is indicated for:
- the treatment of adult men with metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy (see section 5. 1).
- the treatment of adult men with high-risk non-metastatic castration-resistant prostate cancer (CRPC) (see section 5. 1).
- the treatment of adult men with metastatic CRPC who are asymptomatic or mildly symptomatic after failure of androgen deprivation therapy in whom chemotherapy is not yet clinically indicated (see section 5. 1).
- the treatment of adult men with metastatic CRPC whose disease has progressed on or after docetaxel therapy.
Resitance to enzalutamide therapy has been observed. This may occurred due to an upregulation of NF-B2/p52.
L02BB04
L02BB04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02B - Hormone antagonists and related agents
L02BB - Anti-androgens
L02BB04 - Enzalutamide
Absorption
The pharmacokinetic profile of enzalutamide and N-desmethyl enzalutamide (its major active metabolite) is described by a linear two-compartment model with first-order absorption. Enzalutamide also accumulates. Food does not affect its absorption. Tmax, prostate cancer patients = 1 hour (range of 0.5-3 hours); Cmax, steady state, enzalutamide = 16.6 g/mL; Cmax, steady state, N-desmethyl enzalutamide = 12.7 g/mL; Time to steady state, daily dosing = 28 days;
Route of Elimination
Enzalutamide is primarily eliminated by hepatic metabolism. 71% of the dose is recovered in urine (including only trace amounts of enzalutamide and N-desmethyl enzalutamide), and 14% is recovered in feces (0.4% of dose as unchanged enzalutamide and 1% as N-desmethyl enzalutamide).
Volume of Distribution
Apparent volume of distribution (Vd/F), single oral dose = 110 L
Clearance
Apparent clearance (CL/F), single oral dose = 0.56 L/h (range of 0.33 - 1.02 L/h)
Enzalutamide is hepatically metabolized, primarily by CYP2C8 and CYP3A4. The enzyme that converts enzalutamide to its active metabolite, N-desmethyl enzalutamide, is CYP2C8. The activity of N-desmethyl-enzalutamide is similar to that of the parent compound.
The mean terminal half-life (t1/2) for enzalutamide in patients after a single oral dose is 5.8 days (range 2.8 to 10.2 days). Following a single 160 mg oral dose of enzalutamide in healthy volunteers, the mean terminal t1/2 for N-desmethyl enzalutamide is approximately 7.8 to 8.6 days.
Enzalutamide is a competitive androgen receptor inhibitor that effects multiple stages of the signalling pathway. It is able to inhibit androgen binding to its receptor, androgen receptor nuclear translocation, and subsequent interaction with DNA. As a result, proliferation of prostate cancer cells decreases which ultimately leads to apoptosis and decreased tumour volume.
