
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. C.m.9155
2. Env905
3. Epitopic
1. 23674-86-4
2. Durezol
3. Dfba
4. Myser
5. Epitopic
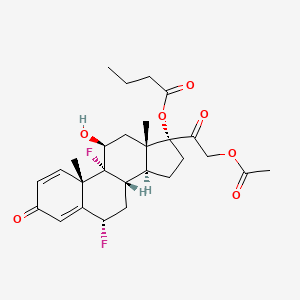
6. [(6s,8s,9r,10s,11s,13s,14s,17r)-17-(2-acetyloxyacetyl)-6,9-difluoro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] Butanoate
7. Difluoroprednisolone Butyrate Acetate
8. W 6309
9. Cm 9155
10. S8a06qg2qe
11. Difluprednatum
12. Mls000028663
13. Mls001148580
14. Chebi:31485
15. W-6309
16. Smr000058924
17. (6alpha,11beta)-21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)pregna-1,4-diene-3,20-dion
18. Difluprednato
19. 6alpha,9alpha-difluoroprednisolone 21-acetate 17-butyrate
20. Durezol (tn)
21. Unii-s8a06qg2qe
22. Difluprednatum [inn-latin]
23. Difluprednato [inn-spanish]
24. Difluprednate [usan:inn:jan]
25. Ncgc00168749-01
26. Einecs 245-815-4
27. Mfcd00214273
28. Opera_id_1287
29. Difluprednate [mi]
30. Difluprednate [inn]
31. Difluprednate [jan]
32. Schembl4580
33. Difluprednate [usan]
34. Dsstox_cid_26773
35. Dsstox_rid_81894
36. Dsstox_gsid_46773
37. Difluprednate [vandf]
38. Mls001333701
39. St60-1
40. Difluprednate [mart.]
41. Difluprednate [who-dd]
42. Gtpl7474
43. Chembl1201749
44. Difluprednate (jan/usan/inn)
45. Dtxsid0046773
46. 6-alpha,9-alpha-difluoroprednisolone 17-butyrate 21-acetate
47. Hms2231d18
48. Difluprednate [orange Book]
49. (6s,8s,9r,10s,11s,13s,14s,17r)-17-(2-acetoxyacetyl)-6,9-difluoro-11-hydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-17-yl Butyrate
50. Act03289
51. Zinc4212945
52. Tox21_112628
53. S4095
54. Ccg-269762
55. Db06781
56. 6alpha,9-difluoro-11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate
57. 21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)pregna-1,4-diene-3,20-dione (6alpha,11beta)-
58. As-15800
59. Hy-17569
60. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)-, (6.alpha.,11.beta.)-
61. Cas-23674-86-4
62. D5579
63. D01266
64. Ab00383058_10
65. 674d864
66. A932746
67. Q736113
68. Sr-01000000265
69. Q-101389
70. Sr-01000000265-4
71. 6alpha-9-difluoroprednisolone 21-acetate 17-butyrate
72. 6 Alpha ,9 Alpha -difluoroprednisolone 21-acetate 17-butyrate
73. 6alpha,9alpha-difluoroprednisolone 21-acetate 17-butyrate, >=98%
74. (1r,2s,8s,10s,11s,14r,15s,17s)-14-[2-(acetyloxy)acetyl]-1,8-difluoro-17-hydroxy-2,15-dimethyl-5-oxotetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-14-yl Butanoate
75. 6.alpha.,9-difluoro-11.beta.,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate
76. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)-, (6alpha,11beta)-
77. Pregna-1,4-diene-3,20-dione, 6-alpha,9-difluoro-11-beta,17,21-trihydroxy-, 21-acetate, 17-butyrate
78. Pregna-1,4-diene-3,20-dione,21-(acetyloxy)-6,9-difluoro-11-hydroxy-17-(1-oxobutoxy)-, (6a,11b)-
| Molecular Weight | 508.5 g/mol |
|---|---|
| Molecular Formula | C27H34F2O7 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 508.22725974 g/mol |
| Monoisotopic Mass | 508.22725974 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Durezol |
| PubMed Health | Difluprednate (Into the eye) |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Durezol (difluprednate ophthalmic emulsion) 0.05% is a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. The chemical name is 6,9difluoro-11,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate (CAS number 23674-... |
| Active Ingredient | Difluprednate |
| Dosage Form | Emulsion |
| Route | Ophthalmic |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Alcon Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Durezol |
| PubMed Health | Difluprednate (Into the eye) |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Durezol (difluprednate ophthalmic emulsion) 0.05% is a sterile, topical anti-inflammatory corticosteroid for ophthalmic use. The chemical name is 6,9difluoro-11,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 17-butyrate (CAS number 23674-... |
| Active Ingredient | Difluprednate |
| Dosage Form | Emulsion |
| Route | Ophthalmic |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Alcon Pharms |
For the treatment of inflammation and pain associated with ocular surgery.
FDA Label
Difluprednate is a corticosteroid used as an anti-inflammatory steroidal drug used primarily in ocular surgery.
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AC - Corticosteroids, potent (group iii)
D07AC19 - Difluprednate
Absorption
Difluprednate penetrates the corneal epithelium rapidly and effectively. Low systemic absorption.
Route of Elimination
78.5% of radioactivity was excreted aftert 24 hours, and 99.5% by 7 days after a single dose of labeled difluprednate instilled in the right eyes of pigmented rabbits.
Difluprednate is rapidly deacetylated in the aqueous humor to difluoroprednisolone butyrate (DFB), the drugs active metabolite. Endogenous tissue esterases then metabolize DFB to the inert metabolite hydroxyfluoroprednisolone butyrate (HFB), which limits systemic exposure to the active compound.
Corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins (lipocortins). It is postulated that these proteins control the biosynthesis of potent mediators of infammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2014-08-27
Pay. Date : 2014-04-14
DMF Number : 25228
Submission : 2011-08-23
Status : Active
Type : II

Registration Number : 301MF10046
Registrant's Address : Via Pavia, 1-27027 Gropello Cairoli, Pavia, Italy
Initial Date of Registration : 2019-08-20
Latest Date of Registration :

NDC Package Code : 46439-8738
Start Marketing Date : 2011-07-01
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : EU |
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

NDC Package Code : 46439-8739
Start Marketing Date : 2011-07-01
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : EU |

Date of Issue : 2024-11-05
Valid Till : 2027-11-04
Written Confirmation Number : WC-0485
Address of the Firm :





NDC Package Code : 26251-0003
Start Marketing Date : 2017-12-06
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE
55
PharmaCompass offers a list of Difluprednate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Difluprednate manufacturer or Difluprednate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Difluprednate manufacturer or Difluprednate supplier.
PharmaCompass also assists you with knowing the Difluprednate API Price utilized in the formulation of products. Difluprednate API Price is not always fixed or binding as the Difluprednate Price is obtained through a variety of data sources. The Difluprednate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Durezol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Durezol, including repackagers and relabelers. The FDA regulates Durezol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Durezol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Durezol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Durezol supplier is an individual or a company that provides Durezol active pharmaceutical ingredient (API) or Durezol finished formulations upon request. The Durezol suppliers may include Durezol API manufacturers, exporters, distributors and traders.
click here to find a list of Durezol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Durezol DMF (Drug Master File) is a document detailing the whole manufacturing process of Durezol active pharmaceutical ingredient (API) in detail. Different forms of Durezol DMFs exist exist since differing nations have different regulations, such as Durezol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Durezol DMF submitted to regulatory agencies in the US is known as a USDMF. Durezol USDMF includes data on Durezol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Durezol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Durezol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Durezol Drug Master File in Japan (Durezol JDMF) empowers Durezol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Durezol JDMF during the approval evaluation for pharmaceutical products. At the time of Durezol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Durezol suppliers with JDMF on PharmaCompass.
A Durezol written confirmation (Durezol WC) is an official document issued by a regulatory agency to a Durezol manufacturer, verifying that the manufacturing facility of a Durezol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Durezol APIs or Durezol finished pharmaceutical products to another nation, regulatory agencies frequently require a Durezol WC (written confirmation) as part of the regulatory process.
click here to find a list of Durezol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Durezol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Durezol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Durezol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Durezol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Durezol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Durezol suppliers with NDC on PharmaCompass.
Durezol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Durezol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Durezol GMP manufacturer or Durezol GMP API supplier for your needs.
A Durezol CoA (Certificate of Analysis) is a formal document that attests to Durezol's compliance with Durezol specifications and serves as a tool for batch-level quality control.
Durezol CoA mostly includes findings from lab analyses of a specific batch. For each Durezol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Durezol may be tested according to a variety of international standards, such as European Pharmacopoeia (Durezol EP), Durezol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Durezol USP).

