
Synopsis
Synopsis
0
KDMF
0
VMF
0
FDA Orange Book
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
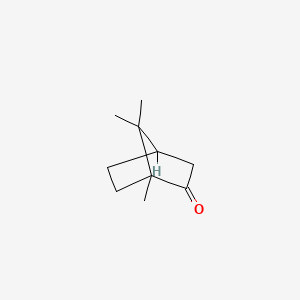

1. Camphor, (+-)-isomer
2. Camphor, (1r)-isomer
3. Camphor, (1s)-isomer
1. 76-22-2
2. 2-camphanone
3. Dl-camphor
4. (+/-)-camphor
5. 2-bornanone
6. 1,7,7-trimethylbicyclo[2.2.1]heptan-2-one
7. (+)-camphor
8. Bornan-2-one
9. Root Bark Oil
10. Kampfer
11. 21368-68-3
12. Spirit Of Camphor
13. 2-camphonone
14. Gum Camphor
15. 464-48-2
16. L-(-)-camphor
17. 1,7,7-trimethylnorcamphor
18. Alphanon
19. 1,7,7-trimethylbicyclo[2.2.1]-2-heptanone
20. D-(+)-camphor
21. 2-keto-1,7,7-trimethylnorcamphane
22. Formosa Camphor
23. Laurel Camphor
24. Matricaria Camphor
25. Camphor, Synthetic
26. 4,7,7-trimethylbicyclo[2.2.1]heptan-3-one
27. Bornane, 2-oxo-
28. Camphor (synthetic)
29. Huile De Camphre
30. Chebi:36773
31. 2-kamfanon
32. Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-
33. Dl-bornan-2-one
34. (-)-alcanfor
35. Norcamphor, 1,7,7-trimethyl-
36. Camphor, (1r,4r)-(+)-
37. Synthetic Camphor
38. 1,7,7-trimethylbicyclo(2.2.1)heptan-2-one
39. Camphor Oil
40. Camphor Powder
41. Formosa
42. Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (.+/-.)-
43. Japan Camphor
44. Camphor (usp)
45. Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1r)-
46. Racemic Camphor
47. Disperseyellow3
48. Dl-2-bornanone
49. Heet (salt/mix)
50. Dextro,laevo-camphor
51. Sarna (salt/mix)
52. (?)-camphor
53. Dl-camphor (jp17)
54. (.+/-.)-camphor
55. Camphor Powder - Synthetic
56. (1rs,4rs)-1,7,7-trimethylbicyclo(2.2.1)heptan-2-one
57. Dsstox_cid_10955
58. Dsstox_rid_78860
59. Dsstox_gsid_30955
60. Schembl16068
61. Camphor, (.+/-.)-
62. Mls001055495
63. Chembl15768
64. Divk1c_000724
65. Gtpl2422
66. Dtxsid5030955
67. Hms502e06
68. Kbio1_000724
69. Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-, (1s)-
70. Ninds_000724
71. Hms2268a06
72. Hms3885j06
73. Hy-n0808
74. Tox21_200237
75. Bbl012963
76. Mfcd00074738
77. S3851
78. S4516
79. Stk803534
80. ( Inverted Exclamation Marka)-camphor
81. Akos000118728
82. Akos022060577
83. Ac-5284
84. Ccg-266237
85. Ccg-266238
86. Db14156
87. Lmpr0102120001
88. Un 2717
89. Cas-76-22-2
90. Idi1_000724
91. Ncgc00090681-05
92. Ncgc00090730-01
93. Ncgc00090730-02
94. Ncgc00090730-05
95. Ncgc00257791-01
96. Ac-15523
97. Smr000386909
98. Vs-03622
99. (1r,4r)-1,7,7-trimethylnorbornan-2-one
100. Db-051377
101. Db-056037
102. Db-070734
103. C1251
104. Cs-0009813
105. Ft-0607017
106. Ft-0607018
107. Ft-0608303
108. 4,7,7-trimethyl-3-bicyclo[2.2.1]heptanone
109. 1,7,7-trimethyl-bicyclo[2.2.1]heptan-6-one
110. C00809
111. C18369
112. D00098
113. E75814
114. 1,7,7-trimethyl-bicyclo[2.2.1]heptan-2-one
115. A838646
116. Q181559
117. Q-200784
118. W-109539
119. W-110530
120. (+/-)-1,7,7-trimethyl-bicyclo[2,2,1]heptane-2-one
121. F0001-0763
122. Z940713494
| Molecular Weight | 152.23 g/mol |
|---|---|
| Molecular Formula | C10H16O |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 152.120115130 g/mol |
| Monoisotopic Mass | 152.120115130 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 217 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents, Local
National Library of Medicine's Medical Subject Headings online file (MeSH, 2014); Available from, as of July 31, 2014: https://www.nlm.nih.gov/cgi/mesh/2014/MB_cgi?term=Camphor
It /SRP: was formerly/ used ... In humans for inflamed joints, sprains, and rheumatic and other inflammatory conditions such as colds in throat and chest. ... patient may feel improved /however/ inflammation is not affected.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 724
MEDICATION (VET): Locally, camphor is weakly antiseptic and has a rubefacient action when applied to skin. It is a common ingredient of many liniments.
Jones, L.M., et al. Veterinary Pharmacology & Therapeutics. 4th ed. Ames: Iowa State University Press, 1977., p. 414
MEDICATION (VET): /Camphor/ ... As a steam inhalant has been popular in respiratory diseases of horses and poultry. Orally, it has been popular in antiferment and carminative mixtures for calf scours, and in expectorant mixtures.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 74
For more Therapeutic Uses (Complete) data for CAMPHOR (10 total), please visit the HSDB record page.
Keep pharmaceutical preparations and moth-repellents out of the reach of children and irresponsible people. Label camphorated oil appropriately to avoid mistaking it for castor oil and cough syrups. Do not place camphor ointments into infants' nostrils. Do not use the rubefacient on abraded skin.
IPCS; Poisons Information Monograph 095: Camphor. (Date of last update: May 1989). Available from, as of June 30, 2014: https://www.inchem.org/documents/pims/pharm/camphor.htm#SectionTitle:1.5%20Brand%20names,%20Trade%20na
Camphor and camphor containing products should be avoided in children who have a history of febrile convulsions or other predisposing factors for convulsions.
National Poisons Information Service Center, United Kingdom; Poisons Information Monograph: Camphor. (March 1996). Available from, as of June 30, 2014: https://www.inchem.org/documents/ukpids/ukpids/ukpid19.htm
Camphor should not be applied to the nostrils of infants even in small quantities, as this may cause immediate collapse.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 2273
The UK Committee on the Review of Medicines recommended that camphor should not be included in products intended for the treatment of hepatic and biliary disorders, gallstones, colic, renal disorders, urinary tract infections, or ureteral stones. The use of camphor parenterally or as irrigants was considered undesirable due to the associated safety hazard.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 2273
For more Drug Warnings (Complete) data for CAMPHOR (11 total), please visit the HSDB record page.
...fatalities in children have been recorded from 1g.
SWEETMAN, S.C. (ed.) Martindale-The Complete Drug Reference. 36th ed. London: The Pharmaceutical Press, 2009., p. 2273
...The literature from 1964-1983 /was reviewed/, and... the mean dose ingested by patients with major symptoms /was/ 124 mg/kg, with the mean dose in fatal cases being 199 mg/kg. /It was/... suggested, based on /the/ analysis of these figures, that patients ingesting <10 mg/kg of camphor and displaying no symptoms required no treatment. Adults have survived ingestions of up to 42 g, but usually doses in excess of 2 g produce dangerous effects. Fatal doses in children have ranged from 0.7-1.0 g.
National Poisons Information Service Center, United Kingdom; Poisons Information Monograph: Camphor. (March 1996). Available from, as of June 30, 2014: https://www.inchem.org/documents/ukpids/ukpids/ukpid19.htm
Dosages exceeding 2.0 grams have been reported to be /CNS depressant/ and may cause convulsions, delirium, hallucinations, and death.
McGuffin M, Hobbs C et al., eds; American Herbal Products Association's Botanical Safety Handbook. p. 31 (1997)
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
Camphor is rapidly absorbed from the mucous membranes and the gastrointestinal tract. ... It is also absorbed through inhalation, through dermal application, and by nasal instillation.
Ford MD, Delaney KA, Ling LJ, Erickson T; Clinical Toxicology. W.B. Saunders Company., Philadelphia, PA. 2001, p. 339
... after camphor ingestion by mothers ... camphor was detectable in maternal blood 15 min after ingestion, but not after 8 hr. At delivery 36 hr later ... it was present in amnionic fluid, cord and fetal blood and fetal brain, liver and kidneys.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. III-85
Alimentary absorption of pure camphor, or of alcohol solution ... is quite rapid, but from the oil preparation absorption is constant. Camphor is ... slowly absorbed from sc or im depots.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 16
Absorbed camphor is mainly eliminated as the oxidized camphorol in the urine, although some appears in the breath, sweat, and feces.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 74
For more Absorption, Distribution and Excretion (Complete) data for CAMPHOR (7 total), please visit the HSDB record page.
In the liver microsomes of female mice, two induction phases during inhalation of DL-camphor were found. During the first 24 hr the apparent molar activity of the ethylumbelliferone dealkylase decreased. In the second phase, the molar ethylumbelliferone dealkylase activity was constant.
Mohn G; Different phases of hydroxylase induction in liver microsomes of female mice during inhalation of cyclohexane and D,L-camphor; Microsomes Drug Oxid, Proc Int Symp, 3rd: 59-66 (1977)
The metabolism of (+)-camphor and (-)-camphor was investigated in rabbits after administration /through/ stomach tube; metabolites of (+)-camphor were (+)-borneol, (+)-5-endo-hydroxycamphor, and (+)-3-endohydroxycamphor.
ROBERTSON JS, HUSSAIN M; METABOLISM OF CAMPHORS AND RELATED COMPOUNDS; BIOCHEM J 113 (1): 57-65 (1969)
Camphor is rapidly oxidized to campherols (2-hydroxycamphor and 3-hydroxycamphor), and then conjugated in the liver to the glucuronide form. Camphor-related metabolites are relatively fat-soluble and may accumulate in fatty tissue.
IPCS; Poisons Information Monograph 095: Camphor. (Date of last update:May 1989). Available from, as of June 30, 2014: https://www.inchem.org/documents/pims/pharm/camphor.htm#SectionTitle:1.5%20Brand%20names,%20Trade%20na
167 minutes (200 mg camphor ingested alone); 93 minutes (200 mg camphor ingested with a solvent - Tween 80).
National Poisons Information Service Center, United Kingdom; Poisons Information Monograph: Camphor. (March 1996). Available from, as of June 30, 2014: https://www.inchem.org/documents/ukpids/ukpids/ukpid19.htm
Camphor is a naturally occurring compound that is used as a major active ingredient of balms and liniments supplied as topical analgesics. ... Capsaicin and menthol, two other topically applied agents widely used for similar purposes, are known to excite and desensitize sensory nerves by acting on two members of transient receptor potential (TRP) channel superfamily: heat-sensitive TRP vanilloid subtype 1 (TRPV1) and cold-sensitive TRP channel M8, respectively. Camphor has recently been shown to activate TRPV3, and here /investigators/ show that camphor also activates heterologously expressed TRPV1, requiring higher concentrations than capsaicin. Activation was enhanced by phospholipase C-coupled receptor stimulation mimicking inflamed conditions. Similar camphor-activated TRPV1-like currents were observed in isolated rat DRG neurons and were strongly potentiated after activation of protein kinase C with phorbol-12-myristate-13-acetate. Camphor activation of rat TRPV1 was mediated by distinct channel regions from capsaicin, as indicated by camphor activation in the presence of the competitive inhibitor capsazepine and in a capsaicin-insensitive point mutant. Camphor did not activate the capsaicin-insensitive chicken TRPV1. TRPV1 desensitization is believed to contribute to the analgesic actions of capsaicin. /The authors/ found that, although camphor activates TRPV1 less effectively, camphor application desensitized TRPV1 more rapidly and completely than capsaicin. Conversely, TRPV3 current sensitized after repeated camphor applications, which is inconsistent with the analgesic role of camphor. /Investigators/ also found that camphor inhibited several other related TRP channels, including ankyrin-repeat TRP 1 (TRPA1). The camphor-induced desensitization of TRPV1 and block of TRPA1 may underlie the analgesic effects of camphor.
PMID:16192383 Xu H et al; J Neurosci. 25(39):8924-37 (2005).

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
66
PharmaCompass offers a list of Camphor API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Camphor manufacturer or Camphor supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Camphor manufacturer or Camphor supplier.
PharmaCompass also assists you with knowing the Camphor API Price utilized in the formulation of products. Camphor API Price is not always fixed or binding as the Camphor Price is obtained through a variety of data sources. The Camphor Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Camphor, synthetic manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Camphor, synthetic, including repackagers and relabelers. The FDA regulates Camphor, synthetic manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Camphor, synthetic API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Camphor, synthetic manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Camphor, synthetic supplier is an individual or a company that provides Camphor, synthetic active pharmaceutical ingredient (API) or Camphor, synthetic finished formulations upon request. The Camphor, synthetic suppliers may include Camphor, synthetic API manufacturers, exporters, distributors and traders.
click here to find a list of Camphor, synthetic suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Camphor, synthetic DMF (Drug Master File) is a document detailing the whole manufacturing process of Camphor, synthetic active pharmaceutical ingredient (API) in detail. Different forms of Camphor, synthetic DMFs exist exist since differing nations have different regulations, such as Camphor, synthetic USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Camphor, synthetic DMF submitted to regulatory agencies in the US is known as a USDMF. Camphor, synthetic USDMF includes data on Camphor, synthetic's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Camphor, synthetic USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Camphor, synthetic suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Camphor, synthetic Drug Master File in Japan (Camphor, synthetic JDMF) empowers Camphor, synthetic API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Camphor, synthetic JDMF during the approval evaluation for pharmaceutical products. At the time of Camphor, synthetic JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Camphor, synthetic suppliers with JDMF on PharmaCompass.
A Camphor, synthetic CEP of the European Pharmacopoeia monograph is often referred to as a Camphor, synthetic Certificate of Suitability (COS). The purpose of a Camphor, synthetic CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Camphor, synthetic EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Camphor, synthetic to their clients by showing that a Camphor, synthetic CEP has been issued for it. The manufacturer submits a Camphor, synthetic CEP (COS) as part of the market authorization procedure, and it takes on the role of a Camphor, synthetic CEP holder for the record. Additionally, the data presented in the Camphor, synthetic CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Camphor, synthetic DMF.
A Camphor, synthetic CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Camphor, synthetic CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Camphor, synthetic suppliers with CEP (COS) on PharmaCompass.
A Camphor, synthetic written confirmation (Camphor, synthetic WC) is an official document issued by a regulatory agency to a Camphor, synthetic manufacturer, verifying that the manufacturing facility of a Camphor, synthetic active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Camphor, synthetic APIs or Camphor, synthetic finished pharmaceutical products to another nation, regulatory agencies frequently require a Camphor, synthetic WC (written confirmation) as part of the regulatory process.
click here to find a list of Camphor, synthetic suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Camphor, synthetic as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Camphor, synthetic API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Camphor, synthetic as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Camphor, synthetic and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Camphor, synthetic NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Camphor, synthetic suppliers with NDC on PharmaCompass.
Camphor, synthetic Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Camphor, synthetic GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Camphor, synthetic GMP manufacturer or Camphor, synthetic GMP API supplier for your needs.
A Camphor, synthetic CoA (Certificate of Analysis) is a formal document that attests to Camphor, synthetic's compliance with Camphor, synthetic specifications and serves as a tool for batch-level quality control.
Camphor, synthetic CoA mostly includes findings from lab analyses of a specific batch. For each Camphor, synthetic CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Camphor, synthetic may be tested according to a variety of international standards, such as European Pharmacopoeia (Camphor, synthetic EP), Camphor, synthetic JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Camphor, synthetic USP).

