
Synopsis
Synopsis
0
EU WC
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Pyridoxin
2. Pyridoxine
3. Pyridoxol
4. Pyridoxol Hydrochloride
5. Rodex
1. 58-56-0
2. Pyridoxine Hcl
3. Pyridoxol Hydrochloride
4. Vitamin B6
5. Vitamin B6 Hydrochloride
6. Pyridoxine Chloride
7. Hexa-betalin
8. Alestrol
9. Becilan
10. Benadon
11. Hexavibex
12. Hexermin
13. Hexobion
14. Pyridipca
15. Beesix
16. Pydox
17. Adermine Hydrochloride
18. Campoviton 6
19. Aderoxine
20. Pyridoxinium Chloride
21. Aderomine Hydrochloride
22. Hexabione Hydrochloride
23. Pyridoxin Hydrochloride
24. Pyridoxinum Hydrochloricum
25. 4,5-bis(hydroxymethyl)-2-methylpyridin-3-ol Hydrochloride
26. Bendectin
27. 3,4-pyridinedimethanol, 5-hydroxy-6-methyl-, Hydrochloride
28. Pyridoxol, Hydrochloride
29. Pyridoxine (hydrochloride)
30. 5-hydroxy-6-methyl-3,4-pyridinedimethanol Hydrochloride
31. Mfcd00012807
32. 2-methyl-3-hydroxy-4,5-bis(hydroxymethyl)pyridine Hydrochloride
33. 3-hydroxy-4,5-dimethylol-alpha-picoline Hydrochloride
34. 5-hydroxy-6-methyl-3,4-pyridinedicarbinol Hydrochloride
35. 4,5-bis(hydroxymethyl)-2-methylpyridin-3-ol;hydrochloride
36. Bonasanit
37. (5-hydroxy-6-methylpyridine-3,4-diyl)dimethanol Hydrochloride
38. Nsc-36225
39. Adermin Hydrochloride
40. 5-hydroxy-6-methyl-3,4-pyridinedimethanol, Hydrochloride
41. 68y4cf58bv
42. Hexabetalin
43. Aderoxin
44. Godabion
45. Paxadon
46. Pyridox
47. Chebi:30961
48. Pyridoxine Hydrogen Chloride
49. 8064-77-5
50. Hexa-betalin (r)
51. Spondylonal
52. Rodex (r)
53. Pn Hcl
54. Pyridoxin Hydrochloride (vitamin B6 Hydrochloride)
55. Pyridoxine Monohydrochloride
56. Pyridoxol (hydrochloride);vitamin B6 (hydrochloride)
57. 4,5-bis(hydroxymethyl)-2-methyl-3-pyridinol Hydrochloride
58. Pyridoxine, Hydrochloride
59. 3,4-pyridinedimethanol, 5-hydroxy-6-methyl-, Hydrochloride (1:1)
60. Pyridoxinum Hydrochloricum (hungarian)
61. Smr000674613
62. Hexa-betalin (tn)
63. Nsc36225
64. Ccris 1903
65. Component Of Alestrol
66. Hsdb 1212
67. Tex Six T.r.
68. Clorhidrato De Piridoxina
69. Ncgc00164317-01
70. Vitamin B6-hydrochloride
71. Einecs 200-386-2
72. Nsc 36225
73. Unii-68y4cf58bv
74. Ai3-19016
75. Pyridoxine Hydrochloride [jan]
76. 3-hydroxy-4,5-dihydroxymethyl-2-methylpyridine Hydrochloride
77. Prestwick_925
78. Pyridoxine Hydrochloride [usp:jan]
79. Pyridoxini Hydrochloridum
80. 4,5-bis(hydroxymethyl)-3-hydroxy-2-methylpyridine Hydrochloride
81. Pyridoxol (hydrochloride)
82. Vitamin B6, Hydrochloride
83. Vitamin B6 (hydrochloride)
84. Dsstox_cid_10780
85. Dsstox_rid_79601
86. Dsstox_gsid_40792
87. Schembl42293
88. Mls001074329
89. Mls002153915
90. Pyridoxine Hcl [inci]
91. Pyridoxine Hcl [vandf]
92. Pyridoxine Hydrochloride, 98%
93. 3-hydroxy-4,5-dimethylol-.alpha.-picoline Hydrochloride
94. Chembl1200756
95. Dtxsid1040792
96. Hms1569n08
97. Amy37035
98. Hy-n0682
99. Tox21_302476
100. Pyridoxine Hydrochloride [mi]
101. S3113
102. 3-hydroxy-4,5-dimethylol-alpha-pic
103. Pyridoxine Hydrochloride (jp17/usp)
104. Pyridoxine Hydrochloride [fcc]
105. Akos015891679
106. Pyridoxine Hcl (vitamin B6) Solution
107. Ccg-220623
108. Cs-w020418
109. Pyridoxine Hydrochloride [hsdb]
110. 3, 5-hydroxy-6-methyl-, Hydrochloride
111. Pyridoxine Hydrochloride [mart.]
112. Pyridoxine Hydrochloride [vandf]
113. Ncgc00180946-01
114. Ncgc00256911-01
115. Pyridoxine Hydrochloride [usp-rs]
116. Pyridoxine Hydrochloride [who-dd]
117. Pyridoxine Hydrochloride [who-ip]
118. Pyridoxinum Hydrochloricum [hpus]
119. Ac-12024
120. As-11752
121. Sy061587
122. Wln: T6nj B1 Cq D1q E1q &gh
123. Pyridoxine Hydrochloride, >=98% (hplc)
124. Db-053225
125. Pyridoxine Hydrochloride, P.a., 98-102%
126. B2017
127. Ft-0631260
128. P0561
129. Pyridoxine Hydrochloride [green Book]
130. Sw197042-3
131. Tpn Component Pyridoxine Hydrochloride
132. Pyridoxine Hydrochloride [ep Impurity]
133. Pyridoxine Hydrochloride [orange Book]
134. D02179
135. H12039
136. Pyridoxine Hydrochloride [ep Monograph]
137. Pyridoxine Hydrochloride [usp Monograph]
138. Pyridoxine Hydrochloride Component Of Tpn
139. Pyridoxini Hydrochloridum [who-ip Latin]
140. Vitamin B6 (as Pyridoxine Hydrochloride)
141. Bendectin Component Pyridoxine Hydrochloride
142. Bonjesta Component Pyridoxine Hydrochloride
143. Diclectin Component Pyridoxine Hydrochloride
144. Diclegis Component Pyridoxine Hydrochloride
145. Pyridoxine Hydrochloride (b6), Analytical Standard
146. Pyridoxine Hydrochloride, Plant Cell Culture Tested
147. Q-201647
148. Sr-05000001644-4
149. Pyridoxine Hydrochloride Component Of Bendectin
150. Pyridoxine Hydrochloride Component Of Bonjesta
151. Q26841284
152. F0001-2400
153. Pyridoxine Hydrochloride, Saj Special Grade, >=98.0%
154. Pyridoxine Hydrochloride, Vetec(tm) Reagent Grade, 98%
155. Pyridoxine Hydrochloride, Meets Usp Testing Specifications
156. Pyridoxine Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
157. 3-hydroxy-4,5-bis(hydroxymethyl)-2-methylpyridine Hydrochloride
158. Pyridoxine Hydrochloride, Certified Reference Material, Tracecert(r)
159. Pyridoxine Hydrochloride, British Pharmacopoeia (bp) Reference Standard
160. Pyridoxine Hydrochloride, European Pharmacopoeia (ep) Reference Standard
161. Pyridoxin Hydrochloride (vitamin B6 Hydrochloride) 100 Microg/ml In Methanol
162. Pyridoxine Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
163. Pyridoxine Hydrochloride, United States Pharmacopeia (usp) Reference Standard
164. Pyridoxine Hcl (vitamin B6) Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
165. Pyridoxine Hydrochloride, Bioreagent, Suitable For Cell Culture, Suitable For Insect Cell Culture, Suitable For Plant Cell Culture
166. Pyridoxine Hydrochloride, Pharmagrade, Ep, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production
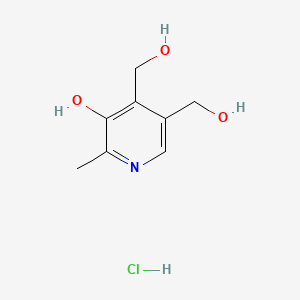
| Molecular Weight | 205.64 g/mol |
|---|---|
| Molecular Formula | C8H12ClNO3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 205.0505709 g/mol |
| Monoisotopic Mass | 205.0505709 g/mol |
| Topological Polar Surface Area | 73.6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 142 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Pyridoxine hydrochloride |
| Drug Label | Pyridoxine Hydrochloride Injection, USP is a sterile solution of pyridoxine hydrochloride in Water for Injection. Each mL contains 100 mg pyridoxine hydrochloride and 0.5% chlorobutanol anhydrous (chloral deriv.). pH adjusted with sodium hydroxid... |
| Active Ingredient | Pyridoxine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 100mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Pyridoxine hydrochloride |
| Drug Label | Pyridoxine Hydrochloride Injection, USP is a sterile solution of pyridoxine hydrochloride in Water for Injection. Each mL contains 100 mg pyridoxine hydrochloride and 0.5% chlorobutanol anhydrous (chloral deriv.). pH adjusted with sodium hydroxid... |
| Active Ingredient | Pyridoxine hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 100mg/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
DOSE: USUAL, ORAL OR PARENTERAL, PROPHYLACTIC, 2 MG ONCE A DAY; THERAPEUTIC, 5 TO 150 MG DAILY. DOSE FOR MALNUTRITION IS 5 TO 10 MG DAILY; FOR HYPEREMESIS GRAVIDUM & EMESIS OF RADIATION SICKNESS IS 10 TO 100 MG DAILY.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 960
... MAY BE USED PROPHYLACTICALLY TO PREVENT PERIPHERAL NEURITIS IN PATIENTS TREATED WITH ISONIAZID. APPEARS TO AID IN DISSOLUTION OF RENAL STONES OF CALCIUM OXALATE ... INCR EXCRETION OF CALCIUM-CHELATING CITRIC ACID.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 960
MEDICATION (VET): ... ANTIDOTAL IN MICE & DOGS TO CONVULSIVE EFFECTS OF RODENTICIDE CRIMIDINE.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 647
... INFANTS MAY EXHIBIT HYPERIRRITABILITY & EPILEPTIFORM CONVULSIONS DURING FIRST WK OF LIFE THAT PROMPTLY RESPOND TO ADMIN OF PYRIDOXINE. IF UNTREATED, PYRIDOXINE-RESPONSIVE ANEMIA & MENTAL RETARDATION MAY RESULT. /PYRIDOXINE/
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 823
For more Therapeutic Uses (Complete) data for PYRIDOXINE HYDROCHLORIDE (22 total), please visit the HSDB record page.
Pyridoxine is usually nontoxic; however, chronic admin of large dosages has been assoc with adverse neurologic effects. Nausea, headache, paresthesia, somnolence, & incr serum AST (SGOT) & decr serum folic acid concn have been reported. Burning or stinging at the injection site may occur following IM or subcutaneous injection of pyridoxine. Seizures have occurred following IV admin of very large doses. Allergic reactions have been reported occasionally in patients receiving the vitamin.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3319
Pyridoxine should not be used in patients with a history of sensitivity to the vitamin. According to one manufacturer, pyridoxine should not be administered iv to patients with heart disease.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3319
Maternal Medication usually Compatible with Breast-Feeding: B6 (pyridoxine): Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 140 (1994)
Very large doses of pyridoxine have been reported to have a lactation-inhibiting effect. Using oral doses of 600 mg/day, lactation was successfully inhibited in 95% of patients within 1 week as compared to only 17% of placebo-treated controls. /Pyridoxine/
Briggs, G.G, R.K. Freeman, S.J. Yaffe. A Reference Guide to Fetal and Neonatal Risk. Drugs in Pregnancy and Lactation. 4th ed. Baltimore, MD: Williams & Wilkins 1994., p. 751
Vitamin B Complex
A group of water-soluble vitamins, some of which are COENZYMES. (See all compounds classified as Vitamin B Complex.)
THE FAT SOLUBLE VITAMINS A, D, & K ARE ABSORBED FROM SKIN, AS ARE LIPID INSOLUBLE VITAMINS, THIAMINE, RIBOFLAVIN, CALCIUM PANTOTHENATE, PYRIDOXINE. /PYRIDOXINE/
Hayes, W. J., Jr. Toxicology of Pesticides Baltimore: Williams & Wilkins, 1975., p. 149
... READILY ABSORBED FROM GI TRACT. ... EXCRETORY PRODUCT ... OF VITAMIN /WHEN IT IS FED TO MAN IS 4-PYRIDOXIC ACID. /PYRIDOXINE/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1563
Pyridoxine, pyridoxal, and pyridoxamine are readily absorbed from the GI tract following oral administration; however, GI absorption may be diminished in patients with malabsorption syndromes or following gastric resection. Normal serum concentrations of pyridoxine are 30-80 ng/ml.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3318
Vitamin B6 is stored mainly in the liver with lesser amounts in muscle & brain. The total body store of vitamin B6 is estimated to be about 167 mg. Pyridoxal and pyridoxal phosphate, the principal forms of the vitamin present in blood, are highly protein bound. Pyridoxal crosses the placenta, & plasma concn in the fetus are 5 times > maternal plasma concn. The concn of vitamin B6 in milk is about 150 240 mg/mL following maternal intake of 2.5 5 mg of vitamin B6 daily. Following maternal intake of < 2.5 mg of vitamin B6 daily, vitamin B6 concn in milk averages 130 mg/mL.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3318
For more Absorption, Distribution and Excretion (Complete) data for PYRIDOXINE HYDROCHLORIDE (6 total), please visit the HSDB record page.
ALL THREE FORMS OF VITAMIN B6 /PYRIDOXINE, PYRIDOXAL, & PYRIDOXAMINE/ ARE CONVERTED TO PYRIDOXAL PHOSPHATE IN BODY; PYRIDOXAL IS CONVERTED TO PYRIDOXAL PHOSPHATE BY ENZYME, PYRIDOXAL KINASE ... . /PYRIDOXINE/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 1570
/4-PYRIDOXIC ACID/ ... FORMED BY ACTION OF HEPATIC ALDEHYDE OXIDASE ON FREE PYRIDOXAL. ADMIN OF PYRIDOXINE ... RESULTS IN INCR EXCRETION OF PYRIDOXAL ... FIRST TRANSFORMED, DIRECTLY OR INDIRECTLY, TO PYRIDOXAL, WHICH IS THEN OXIDIZED TO 4-PYRIDOXIC ACID ... . /PYRIDOXINE/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 1571
In erythrocytes, pyridoxine is converted to pyridoxal phosphate and pyridoxamine is converted to pyridoxamine phosphate. In the liver, pyridoxine is phosphorylated to pyridoxine phosphate and transaminated to pyridoxal and pyridoxamine which are rapidly phosphorylated. Riboflavin is required for the conversion of pyridoxine phosphate to pyridoxal phosphate. The principal forms of the vitamin in the blood are pyridoxal and pyridoxal phosphate.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3318
In humans, an exogenous source of vitamin B6, is required for amino acid metab; the vitamin is also involved in carbohydrate & lipid metab. Pyridoxine, pyridoxal, & pyridoxamine are converted to the active forms of the vitamin, pyridoxal phosphate & pyridoxamine phosphate, which act as coenzymes in a wide variety of reactions in intermed metab. The active forms of the vitamin are involved in transamination of amino acids & in the conversion of tryptophan to niacin. Pyridoxine appears to be essential in the synth of y aminobutyric acid (GABA) within the CNS & in the synth of heme. Pyridoxine deficiency results in the accum & urinary excretion of xanthurenic acid (an intermed metabolite of tryptophan) & in decr glutamic oxaloacetic transaminase activity in erythrocytes; measurement of either of these may be used to diagnose pyridoxine deficiency. For detn of xanthurenic acid excretion, an oral loading dose of 2 10 g of tryptophan is usually given; urine is collected for 8 hours & then analyzed for xanthurenic acid. Urinary excretion of vitamin B6 or 4 pyridoxic acid can also be analyzed to determine pyridoxine deficiency.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3318
In the liver pyridoxal is oxidized to 4-pyridoxic acid.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3318
TRITIUM-LABELED PYRIDOXINE HYDROCHLORIDE WAS ADMIN TO RATS. ISOTOPE EXCRETION IN URINE OCCURRED WITH HALF-LIFE OF 40-228 DAYS AFTER THE SECOND DAY OF THE EXPT. THE ELIMINATION RATE OF PYRIDOXINE WAS NOT SIGNIFICANTLY AFFECTED BY PYRIDOXINE HYDROCHLORIDE INTAKE.
PMID:5947254 JOHANSSON S ET AL; AM J PHYSIOL 210 (5): 1086 (1966)
Biologic half-life of pyridoxine appears to be 15-20 days.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2000.Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2000 (Plus Supplements)., p. 3319
BRAIN GABA PRODN BEGAN TO RISE 7.5 MIN AFTER IP ADMIN OF 10 MG PYRIDOXINE HYDROCHLORIDE & CONTINUED FOR 40 MIN, THEN DECLINED.
PMID:1193274 ACKROYD B, SMITH WR D; BIOCHEM SOC TRANS 3 (5): 707 (1975)
NUTRITIONAL NEED FOR VITAMIN B6 IS BASED ON ITS COENZYME FUNCTION IN AN UNUSUALLY LARGE NUMBER OF METABOLIC REACTIONS. ... REQUIRED IN METABOLISM OF AMINO ACIDS, FATS & CARBOHYDRATES & FOR SYNTHESIS OF PHYSIOLOGIC REGULATORS SUCH AS NOREPINEPHRINE, SEROTONIN & HISTAMINE.
Furia, T.E. (ed.). CRC Handbook of Food Additives. 2nd ed. Cleveland: The Chemical Rubber Co., 1972., p. 90
PYRIDOXAL PHOSPHATE IS INVOLVED IN SEVERAL METABOLIC TRANSFORMATIONS OF AMINO ACIDS INCLUDING DECARBOXYLATION, TRANSAMINATION, & RACEMIZATION, AS WELL AS ENZYMATIC STEPS IN METABOLISM OF SULFUR-CONTAINING & HYDROXY AMINO ACIDS. /PYRIDOXINE/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1539
PYRIDOXINE ... IS AN ESSENTIAL PART OF THE ENZYME GLYCOGEN PHOSPHORYLASE. /PYRIDOXINE/
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 452
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
 Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.

GDUFA
DMF Review : Reviewed
Rev. Date : 2023-01-23
Pay. Date : 2022-12-28
DMF Number : 37706
Submission : 2023-01-03
Status : Active
Type : II

NDC Package Code : 69037-0075
Start Marketing Date : 1982-01-01
End Marketing Date : 2027-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-04-23
Pay. Date : 2012-11-20
DMF Number : 26544
Submission : 2012-10-30
Status : Active
Type : II

Certificate Number : CEP 2013-165 - Rev 01
Issue Date : 2024-11-07
Type : Chemical
Substance Number : 245
Status : Valid


GDUFA
DMF Review : Reviewed
Rev. Date : 2022-05-09
Pay. Date : 2022-03-11
DMF Number : 36386
Submission : 2021-12-01
Status : Active
Type : II

Certificate Number : CEP 2022-492 - Rev 02
Issue Date : 2025-07-17
Type : Chemical
Substance Number : 245
Status : Valid

Registration Number : 230MF10050
Registrant's Address : Wurmisweg 576, CH-4303, Kaiseraugst, Switzerland
Initial Date of Registration : 2018-04-04
Latest Date of Registration :

NDC Package Code : 63238-5500
Start Marketing Date : 2022-05-10
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : DRUG FOR FURTHER PROCESSING

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7984
Submission : 1989-03-17
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3342
Submission : 1978-09-27
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4506
Submission : 1982-03-18
Status : Inactive
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4388
Submission : 1981-11-25
Status : Inactive
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
GDUFA
DMF Review : Complete
Rev. Date : 2023-01-23
Pay. Date : 2022-12-28
DMF Number : 37706
Submission : 2023-01-03
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4388
Submission : 1981-11-25
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2022-05-09
Pay. Date : 2022-03-11
DMF Number : 36386
Submission : 2021-12-01
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3342
Submission : 1978-09-27
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4506
Submission : 1982-03-18
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-04-23
Pay. Date : 2012-11-20
DMF Number : 26544
Submission : 2012-10-30
Status : Active
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 7984
Submission : 1989-03-17
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1994-003 - Rev 01
Status : Withdrawn by Holder
Issue Date : 2004-10-12
Type : Chemical
Substance Number : 245

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2017-027 - Rev 01
Status : Valid
Issue Date : 2025-07-17
Type : Chemical
Substance Number : 245

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 1998-099 - Rev 03
Status : Valid
Issue Date : 2025-07-17
Type : Chemical
Substance Number : 245

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Pyridoxine Hydrochloride, Grenzach II
Certificate Number : CEP 2022-492 - Rev 02
Status : Valid
Issue Date : 2025-07-17
Type : Chemical
Substance Number : 245

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2013-165 - Rev 01
Status : Valid
Issue Date : 2024-11-07
Type : Chemical
Substance Number : 245

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Registration Number : 218MF11003
Registrant's Address : No. 215, Fengze Road, Tiantai, Zhejiang, People Republic of China
Initial Date of Registration : 2006-12-18
Latest Date of Registration : 2011-06-15

Registration Number : 224MF10016
Registrant's Address : Leping Industrial Zone, Leping, Jiangxi, 333300, People's Republic Of China
Initial Date of Registration : 2012-01-18
Latest Date of Registration : 2013-09-25

Japanese Pharmacopoeia Pyridoxine Hydrochloride
Registration Number : 219MF10118
Registrant's Address : Wurmisweg 576, CH-4303, Kaiseraugst, Switzerland
Initial Date of Registration : 2007-03-23
Latest Date of Registration : 2007-03-23

Japanese Pharmacopoeia Pyridoxine Hydrochloride
Registration Number : 230MF10050
Registrant's Address : Wurmisweg 576, CH-4303, Kaiseraugst, Switzerland
Initial Date of Registration : 2018-04-04
Latest Date of Registration : 2018-04-04

Registration Number : 217MF11117
Registrant's Address : Wurmisweg 576, CH-4303, Kaiseraugst, Switzerland
Initial Date of Registration : 2005-12-05
Latest Date of Registration : 2006-06-09

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Daeshin Pharmaceutical Co., Ltd.
Registration Date : 2025-05-09
Registration Number : 20250509-211-J-1861
Manufacturer Name : Huazhong Pharmaceutical Co.,...
Manufacturer Address : No.118 Xianshan Road, Xiangyang City, Hubei Province, China

Registrant Name : Sanil Pharma Co., Ltd.
Registration Date : 2025-02-11
Registration Number : 20250211-211-J-1758
Manufacturer Name : Hubei Huisheng Pharmaceutica...
Manufacturer Address : 33 Guishan Road, Changjiang Industry Park, Economic Development Area, Xianning City, ...

Registrant Name : IMCD Korea Co., Ltd.
Registration Date : 2020-01-10
Registration Number : 20200110-210-J-525
Manufacturer Name : Jiangxi Tianxin Pharmaceutic...
Manufacturer Address : Leping Industrial Zone, Leping, Jiangxi 333300, China

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
NDC Package Code : 69037-0075
Start Marketing Date : 1982-01-01
End Marketing Date : 2027-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
NDC Package Code : 54077-031
Start Marketing Date : 2012-12-29
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 17337-0423
Start Marketing Date : 2017-02-13
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 11737-300
Start Marketing Date : 2005-01-01
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (0.333kg/kg)
Marketing Category : DRUG FOR FURTHER PROCESSING

NDC Package Code : 51927-5117
Start Marketing Date : 2021-03-31
End Marketing Date : 2027-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN P...

NDC Package Code : 90027-018
Start Marketing Date : 2022-09-01
End Marketing Date : 2027-10-28
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN P...

NDC Package Code : 63238-5500
Start Marketing Date : 2022-05-10
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : DRUG FOR FURTHER PROCESSING

NDC Package Code : 10695-334
Start Marketing Date : 2026-02-10
End Marketing Date : 2027-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN P...

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
NDC Package Code : 83511-038
Start Marketing Date : 2025-12-27
End Marketing Date : 2027-12-31
Dosage Form (Strength) : POWDER (35kg/35kg)
Marketing Category : BULK INGREDIENT

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
NDC Package Code : 49452-6145
Start Marketing Date : 2025-11-19
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : DISCN
Registration Country : USA
DOXYLAMINE SUCCINATE; PYRIDOXINE HYDROCHLORIDE
Brand Name : BENDECTIN
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 10MG;10MG **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1982-01-01
Application Number : 10598
Regulatory Info : DISCN
Registration Country : USA
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : Withdrawn
Registration Country : Malta
Magnesium; Pyridoxine Hydrochloride
Brand Name : Magnevie B6
Dosage Form : Film Coated Tablet
Dosage Strength : 100MG; 10MG
Packaging :
Approval Date : 2009-11-30
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : Withdrawn
Registration Country : Malta
Magnesium; Pyridoxine Hydrochloride
Brand Name : Magnevie B6
Dosage Form : Film Coated Tablet
Dosage Strength : 100MG; 10MG
Packaging :
Approval Date : 2019-08-05
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Beespan Capsules
Dosage Form : CAP
Dosage Strength : 200mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Filibon
Dosage Form : CAP
Dosage Strength : 1u
Packaging : 30X1u
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Minamino
Dosage Form : SYR
Dosage Strength : 1.75mg/5ml
Packaging : 200X1mg/5ml
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Minamino
Dosage Form : SYR
Dosage Strength : 1.75mg/5ml
Packaging : 500X1mg/5ml
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : A-Lennon Vitamin B Complex Injection 10ml
Dosage Form : INJ
Dosage Strength : 50mg/10ml
Packaging : 10X10mg/10ml
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Lennon Vitamin B Complex Injection 2ml
Dosage Form : INJ
Dosage Strength : 10mg/2ml
Packaging : 2X10mg/2ml
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : PYRIDOXINE HYDROCHLORIDE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 100MG/ML
Packaging :
Approval Date : 1982-01-01
Application Number : 80572
Regulatory Info : DISCN
Registration Country : USA
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Syrup
Grade : Oral
Dosage Form : Gel
Grade : Oral, Topical
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Orodispersible Tablet
Grade : Not Available
Application : Chewable & Orodispersible Aids
Excipient Details : Orally Disintegrating Tablets
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Enteric Coatings
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Enteric Coatings
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Cellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Tablet
Grade : Not Available
Application : Coating Systems & Additives
Excipient Details : Immediate Release
Dosage Form : Tablet
Grade : Not Available
Brand Name : SheffCoat™ TF Plus
Application : Coating Systems & Additives
Excipient Details : Titanium Oxide Free Coatings
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Dosage Form : Capsule, Cream / Lotion / Ointment, Emulsion, Gel, Injectable / Parenteral, Suspension, Tablet
Grade : Parenteral, Oral, Topical
Category : Emulsifying Agents, Film Formers & Plasticizers, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Application : Emulsifying Agents, Film Formers & Plasticizers, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Excipient Details : Polysorbate 80 is used as a plasticizer, solubilizer, emulsifier, surfactant, and suspension stabilizer. It is also used in parenteral products.
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Brand Name : EUDRAGIT® L 100-55
Application : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Excipient Details : EUDRAGIT® L 100-55 (powder) is used in delayed release coatings to enhance solubility of poorly soluble drugs such as tablets, capsules & granules.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Methacrylic Acid - Ethyl Acrylate Copolymer
Dosage Form : Granule / Pellet, Tablet
Grade : Oral
Category : Fillers, Diluents & Binders, Granulation, Solubilizers
Application : Parenteral
Excipient Details : L-methionine is used as a supplement in cell culture media, in protein purification processes, and in the production of recombinant proteins.
Pharmacopoeia Ref : USP, EP, BP, JP, ChP
Technical Specs : Low Endotoxin, Low Metals
Ingredient(s) : DL-Methionine Excipient
Dosage Form : Injectable / Parenteral
Grade : Biopharma Grade
Category : Emulsifying Agents, Parenteral
Application : Emulsifying Agents, Parenteral
Excipient Details : Used as a fatty acid in cell culture media in upstream. Also used as an emulsifier and stabilizer in final formulation.
Pharmacopoeia Ref : On Request
Technical Specs : Low bacteria endotoxins, low bioburden (TAMC/TYMC). Customised pa...
Ingredient(s) : Oleic Acid Excipient
Application : Solubilizers
Excipient Details : L-Lysine acetate is used as a solubilizer in capsules along with other oral solid dosage forms.
Pharmacopoeia Ref : NA
Technical Specs : Molecular weight: 206.24 g/mol
Ingredient(s) : L-Lysine Acetate
Application : Parenteral
Excipient Details : L-Methionine is used as an ingredient for cell culture media, and in the production of recombinant proteins.
Pharmacopoeia Ref : On Request
Technical Specs : Low bacteria endotoxins, low bioburden (TAMC/TYMC). Customised pa...
Ingredient(s) : L Methionine
Application : Solubilizers
Excipient Details : L-methionine is used as a solubility enhancer to improve the solubility of poorly soluble drugs such as tablets.
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Tablet
Grade : Parenteral, Oral, Topical
Category : Parenteral, Solubilizers, Topical
Dosage Form : Cream / Lotion / Ointment, Emulsion, Gel, Injectable / Parenteral, Suspension, Tablet
Grade : Parenteral, Oral, Topical
Category : Emulsifying Agents, Parenteral, Solubilizers, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Dosage Form : Cream / Lotion / Ointment, Injectable / Parenteral
Grade : Topical, Parenteral
Category : Parenteral, Topical
Application : Solubilizers
Excipient Details : L-methionine is used as a solubility enhancer to improve the solubility of poorly soluble drugs such as tablets.
Application : Fillers, Diluents & Binders, Taste Masking
Excipient Details : Mannitol is used as a filler, bulking agent and taste masking agent in ODT formulations such as tablets.
Application : Parenteral
Excipient Details : Histidine is used in biotherapeutic formulations and protects monoclonal antibodies in both the liquid and lyophilized state.
Pharmacopoeia Ref : USP, EP, BP, JP, ChP
Technical Specs : Low Endotoxin, Low Metals
Ingredient(s) : L-Histidine Excipient
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Category : Parenteral, Thickeners and Stabilizers
Application : Parenteral, Thickeners and Stabilizers
Excipient Details : Sucrose is used to stabilize proteins, lipids, carbohydrates, ADCs & vaccines. It is also used as a cryopreservative in cell-based bioprocesses.
Pharmacopoeia Ref : USP NF, EP, JP, ChP
Technical Specs : Low Endotoxin, Low Metals
Ingredient(s) : Saccharose Excipient
Dosage Form : Injectable / Parenteral
Grade : Biopharma Grade
Category : Emulsifying Agents, Parenteral
Application : Emulsifying Agents, Parenteral
Excipient Details : Used as a fatty acid in cell culture media in upstream. Also used as an emulsifier and stabilizer in final formulation.
Pharmacopoeia Ref : On Request
Technical Specs : Low bacteria endotoxins, low bioburden (TAMC/TYMC). Customised pa...
Ingredient(s) : Oleic Acid Excipient
Application : Parenteral
Excipient Details : Amino acid used for protein synthesis and cellular metabolism in cell culture media in upstream process.
Pharmacopoeia Ref : On Request
Technical Specs : Low bacteria endotoxins, low bioburden (TAMC/TYMC). Customised pa...
Ingredient(s) : L-Serine
Application : Topical
Excipient Details : L-Serine is used in topical semi-solid formulations such as creams, lotions and gel.
Dosage Form : Injectable / Parenteral
Grade : Biopharma Grade
Category : Parenteral, Thickeners and Stabilizers
Brand Name : Citric Acid Monohydrate
Application : Parenteral, Thickeners and Stabilizers
Excipient Details : Used as a pH regulator and preservative in many Biological formulations and cell culture media. Also it can be used as a chelating agent.
Pharmacopoeia Ref : On Request
Technical Specs : Low bacteria endotoxins, low bioburden (TAMC/TYMC). Customised pa...
Ingredient(s) : Citric Acid Excipient
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
97
PharmaCompass offers a list of Pyridoxine Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pyridoxine Hydrochloride manufacturer or Pyridoxine Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pyridoxine Hydrochloride manufacturer or Pyridoxine Hydrochloride supplier.
PharmaCompass also assists you with knowing the Pyridoxine Hydrochloride API Price utilized in the formulation of products. Pyridoxine Hydrochloride API Price is not always fixed or binding as the Pyridoxine Hydrochloride Price is obtained through a variety of data sources. The Pyridoxine Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Benadon manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Benadon, including repackagers and relabelers. The FDA regulates Benadon manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Benadon API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Benadon manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Benadon supplier is an individual or a company that provides Benadon active pharmaceutical ingredient (API) or Benadon finished formulations upon request. The Benadon suppliers may include Benadon API manufacturers, exporters, distributors and traders.
click here to find a list of Benadon suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Benadon DMF (Drug Master File) is a document detailing the whole manufacturing process of Benadon active pharmaceutical ingredient (API) in detail. Different forms of Benadon DMFs exist exist since differing nations have different regulations, such as Benadon USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Benadon DMF submitted to regulatory agencies in the US is known as a USDMF. Benadon USDMF includes data on Benadon's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Benadon USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Benadon suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Benadon Drug Master File in Japan (Benadon JDMF) empowers Benadon API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Benadon JDMF during the approval evaluation for pharmaceutical products. At the time of Benadon JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Benadon suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Benadon Drug Master File in Korea (Benadon KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Benadon. The MFDS reviews the Benadon KDMF as part of the drug registration process and uses the information provided in the Benadon KDMF to evaluate the safety and efficacy of the drug.
After submitting a Benadon KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Benadon API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Benadon suppliers with KDMF on PharmaCompass.
A Benadon CEP of the European Pharmacopoeia monograph is often referred to as a Benadon Certificate of Suitability (COS). The purpose of a Benadon CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Benadon EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Benadon to their clients by showing that a Benadon CEP has been issued for it. The manufacturer submits a Benadon CEP (COS) as part of the market authorization procedure, and it takes on the role of a Benadon CEP holder for the record. Additionally, the data presented in the Benadon CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Benadon DMF.
A Benadon CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Benadon CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Benadon suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Benadon as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Benadon API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Benadon as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Benadon and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Benadon NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Benadon suppliers with NDC on PharmaCompass.
Benadon Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Benadon GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Benadon GMP manufacturer or Benadon GMP API supplier for your needs.
A Benadon CoA (Certificate of Analysis) is a formal document that attests to Benadon's compliance with Benadon specifications and serves as a tool for batch-level quality control.
Benadon CoA mostly includes findings from lab analyses of a specific batch. For each Benadon CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Benadon may be tested according to a variety of international standards, such as European Pharmacopoeia (Benadon EP), Benadon JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Benadon USP).

