
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
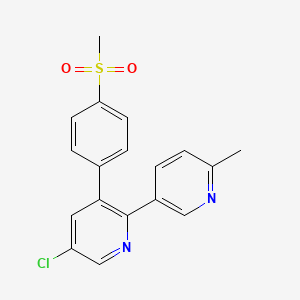

1. Arcoxia
2. L 791456
3. L-791456
4. L791456
5. Mk 0663
6. Mk-0663
7. Mk0663
1. 202409-33-4
2. Arcoxia
3. Tauxib
4. Nucoxia
5. Algix
6. Mk-0663
7. 5-chloro-6'-methyl-3-(4-(methylsulfonyl)phenyl)-2,3'-bipyridine
8. Mk-663
9. L-791456
10. Mk 0663
11. 5-chloro-6'-methyl-3-[4-(methylsulfonyl)phenyl]-2,3'-bipyridine
12. Mk 663
13. 2,3'-bipyridine, 5-chloro-6'-methyl-3-[4-(methylsulfonyl)phenyl]-
14. 5-chloro-2-(6-methylpyridin-3-yl)-3-(4-methylsulfonylphenyl)pyridine
15. Wrx4nfy03r
16. L791456
17. 5-chloro-6'-methyl-3-(p-(methylsulfonyl)phenyl)-2,3'-bipyridine
18. Chebi:6339
19. Chembl416146
20. 2,3'-bipyridine, 5-chloro-6'-methyl-3-(4-(methylsulfonyl)phenyl)-
21. 5-chloro-2-(6-methylpyridin-3-yl)-3-(4-(methylsulfonyl)phenyl)pyridine
22. Ncgc00164578-01
23. Etoricoxibe
24. Etropain
25. Torcoxia
26. Etoxib
27. 5-chloro-3-(4-methanesulfonyl-phenyl)-6'-methyl-[2,3']bipyridinyl
28. Mk-0663;l-791456
29. Dsstox_cid_26457
30. Dsstox_rid_81631
31. Dsstox_gsid_46457
32. Etoricoxib [usan:inn:ban]
33. Kingcox
34. 5-chloro-6'-methyl-3-(4-(methylsulfonyl)-phenyl)-2,3'-bipyridine
35. Etoricoxib [usan]
36. Cas-202409-33-4
37. Sr-05000001486
38. Etoricoxib (usan/inn)
39. Unii-wrx4nfy03r
40. Mk0663
41. Etoricoxibum
42. Etoricoxib- Bio-x
43. Etoricoxib [mi]
44. Etoricoxib [inn]
45. Etoricoxib [mart.]
46. Schembl4680
47. Etoricoxib [who-dd]
48. Gtpl2896
49. Dtxsid3046457
50. Hms2090a05
51. Hms3713p20
52. Hms3885h14
53. Zinc579472
54. Amy30994
55. Bcp06428
56. Ex-a2642
57. Tox21 112206
58. Tox21_112206
59. Bdbm50072064
60. Etoricoxib; Mk-663; Mk-0663
61. Mfcd06797512
62. S4651
63. Akos016010125
64. Tox21_112206_1
65. Ccg-220639
66. Cs-1047
67. Db01628
68. Sb18988
69. Ncgc00164578-02
70. 5ch
71. Ac-29052
72. As-17761
73. Bc164436
74. Etoricoxib 100 Microg/ml In Acetonitrile
75. Hy-15321
76. Db-045133
77. Ft-0602793
78. Ft-0668437
79. D03710
80. Etoricoxib, Vetranal(tm), Analytical Standard
81. Ab01275483-01
82. 409e334
83. A848896
84. L001141
85. Q631202
86. J-013140
87. Sr-05000001486-1
88. Sr-05000001486-2
89. Brd-k54770957-001-01-9
90. Brd-k54770957-001-02-7
91. L-791,456
92. F2173-0490
93. Etoricoxib, United States Pharmacopeia (usp) Reference Standard
94. 5-chloro-3-(4-methanesulfonyl-phenyl)-6''''-methyl-[2,3'''']bipyridinyl
95. 5-chloro-3-(4-methanesulfonyl-phenyl)-6''-methyl-[2,3'']bipyridinyl
96. 5-chloro-3-(4-methanesulfonylphenyl)-2-(6-methylpyridin-3-yl)pyridine
97. 5-chloro-3-[4-(methylsulfonyl)phenyl]-2-(2-methyl-5-pyridinyl)pyridine
98. 5-chloro-6''-methyl-3-[4-(methylsulfonyl)phenyl]-2,3''-bipyridine
| Molecular Weight | 358.8 g/mol |
|---|---|
| Molecular Formula | C18H15ClN2O2S |
| XLogP3 | 3.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 358.0542766 g/mol |
| Monoisotopic Mass | 358.0542766 g/mol |
| Topological Polar Surface Area | 68.3 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 514 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, chronic low back pain, acute pain and gout.
Etoricoxib is a COX-2 selective inhibitor (approximately 106 times more selective for COX-2 inhibition over COX-1).
Cyclooxygenase 2 Inhibitors
A subclass of cyclooxygenase inhibitors with specificity for CYCLOOXYGENASE-2. (See all compounds classified as Cyclooxygenase 2 Inhibitors.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
M01AH05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AH - Coxibs
M01AH05 - Etoricoxib
Absorption
Bioavailability is 100% following oral administration.
Hepatic, primarily via CYP3A4.
Etoricoxib has known human metabolites that include 6-Hydroxymethyletoricoxib and Etoricoxib 1'-N'-oxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
22 hours
Like any other COX-2 selective inhibitor Etoricoxib selectively inhibits isoform 2 of cyclo-oxigenase enzyme (COX-2), preventing production of prostaglandins (PGs) from arachidonic acid.
Date of Issue : 2022-05-18
Valid Till : 2025-05-28
Written Confirmation Number : WC-0019
Address of the Firm :
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34537
Submission : 2020-02-22
Status : Active
Type : II

Date of Issue : 2024-04-03
Valid Till : 2027-01-21
Written Confirmation Number : WC-0493
Address of the Firm :

NDC Package Code : 42765-006
Start Marketing Date : 2020-01-17
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (25kg/25kg)
Marketing Category : BULK INGREDIENT
 Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
Nuray is an expert in the synthesis of Niche novel APIs, the first to launch Generics, NCEs, Advanced Intermediates // USFDA certified.
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
2-Chloro-1,3-bis(dimethylamino)trimethinium hexafl...
CAS Number : 249561-98-6
End Use API : Etoricoxib
About The Company : Zeon Pharma Industries India Pvt ltd is an ISO & GMP certified manufacturer of Bulk drugs & Intermediate and also supplies our associate manufacturing plant API...
4-(methyl sulfonyl)-phenyl acetic acid
CAS Number : 90536-66-6
End Use API : Etoricoxib
About The Company : Zeon Pharma Industries India Pvt ltd is an ISO & GMP certified manufacturer of Bulk drugs & Intermediate and also supplies our associate manufacturing plant API...
CAS Number : 10297-73-1
End Use API : Etoricoxib
About The Company : Zeon Pharma Industries India Pvt ltd is an ISO & GMP certified manufacturer of Bulk drugs & Intermediate and also supplies our associate manufacturing plant API...
4-METHYL SULPHONYLPHENYL ACETIC ACID (4-MSP)
CAS Number : 90536-66-6
End Use API : Etoricoxib
About The Company : Amara Labs Pvt Ltd is a WHO-GMP certified manufacturing company wholly owned by a team of technocrats with working experience of more than 20 years in Multi-Nat...

CAS Number : 5470-70-2
End Use API : Etoricoxib
About The Company : Amara Labs Pvt Ltd is a WHO-GMP certified manufacturing company wholly owned by a team of technocrats with working experience of more than 20 years in Multi-Nat...

2-CHLORO-1,3- BIS(DIMENTYAMINO)TRIMETHINIUMHEXAFLU...
CAS Number : 249561-98-6
End Use API : Etoricoxib
About The Company : Amara Labs Pvt Ltd is a WHO-GMP certified manufacturing company wholly owned by a team of technocrats with working experience of more than 20 years in Multi-Nat...

1-(6-METHYLPYRIDIN-3-YL)-2-[4- (METHYLSULFONYL) PH...
CAS Number : 221615-75-4
End Use API : Etoricoxib
About The Company : Amara Labs Pvt Ltd is a WHO-GMP certified manufacturing company wholly owned by a team of technocrats with working experience of more than 20 years in Multi-Nat...

2-(4-Methanesulfonyl-phenyl)-1- (6-methyl-pyridin-...
CAS Number : 221615-75-4
End Use API : Etoricoxib
About The Company : Anupam Rasayan India Limited was started in 1976 and we are 36 years old. ISO 9001:2008 and ISO 14001:2004 certified chemical company with sound technology, en...

2-ChIoro-1,3-bis(dimethyIamino)trimethinium hexafl...
CAS Number : 291756-76-8
End Use API : Etoricoxib
About The Company : Aptra Synthesis Private Limited, is a reliable and vertically integrated manufacturer of APIs and intermediates, boasting state-of-the-art manufacturing facilit...

4- Methyl sulphonyl phenyl acetic acid
CAS Number : 90536-66-6
End Use API : Etoricoxib
About The Company : Aptra Synthesis Private Limited, is a reliable and vertically integrated manufacturer of APIs and intermediates, boasting state-of-the-art manufacturing facilit...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

Reply
28 Mar 2025
Reply
28 Nov 2024
Reply
14 Nov 2024
Reply
17 Sep 2024
Reply
03 Jan 2024
Reply
25 Oct 2023
Reply
29 Sep 2023
Reply
09 Jun 2023
Reply
12 May 2023
Reply
09 May 2023
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
34
PharmaCompass offers a list of Etoricoxib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Etoricoxib manufacturer or Etoricoxib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Etoricoxib manufacturer or Etoricoxib supplier.
PharmaCompass also assists you with knowing the Etoricoxib API Price utilized in the formulation of products. Etoricoxib API Price is not always fixed or binding as the Etoricoxib Price is obtained through a variety of data sources. The Etoricoxib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A 202409-33-4 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of 202409-33-4, including repackagers and relabelers. The FDA regulates 202409-33-4 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. 202409-33-4 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of 202409-33-4 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A 202409-33-4 supplier is an individual or a company that provides 202409-33-4 active pharmaceutical ingredient (API) or 202409-33-4 finished formulations upon request. The 202409-33-4 suppliers may include 202409-33-4 API manufacturers, exporters, distributors and traders.
click here to find a list of 202409-33-4 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A 202409-33-4 DMF (Drug Master File) is a document detailing the whole manufacturing process of 202409-33-4 active pharmaceutical ingredient (API) in detail. Different forms of 202409-33-4 DMFs exist exist since differing nations have different regulations, such as 202409-33-4 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A 202409-33-4 DMF submitted to regulatory agencies in the US is known as a USDMF. 202409-33-4 USDMF includes data on 202409-33-4's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The 202409-33-4 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of 202409-33-4 suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a 202409-33-4 Drug Master File in Korea (202409-33-4 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of 202409-33-4. The MFDS reviews the 202409-33-4 KDMF as part of the drug registration process and uses the information provided in the 202409-33-4 KDMF to evaluate the safety and efficacy of the drug.
After submitting a 202409-33-4 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their 202409-33-4 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of 202409-33-4 suppliers with KDMF on PharmaCompass.
A 202409-33-4 written confirmation (202409-33-4 WC) is an official document issued by a regulatory agency to a 202409-33-4 manufacturer, verifying that the manufacturing facility of a 202409-33-4 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting 202409-33-4 APIs or 202409-33-4 finished pharmaceutical products to another nation, regulatory agencies frequently require a 202409-33-4 WC (written confirmation) as part of the regulatory process.
click here to find a list of 202409-33-4 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing 202409-33-4 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for 202409-33-4 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture 202409-33-4 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain 202409-33-4 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a 202409-33-4 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of 202409-33-4 suppliers with NDC on PharmaCompass.
202409-33-4 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of 202409-33-4 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right 202409-33-4 GMP manufacturer or 202409-33-4 GMP API supplier for your needs.
A 202409-33-4 CoA (Certificate of Analysis) is a formal document that attests to 202409-33-4's compliance with 202409-33-4 specifications and serves as a tool for batch-level quality control.
202409-33-4 CoA mostly includes findings from lab analyses of a specific batch. For each 202409-33-4 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
202409-33-4 may be tested according to a variety of international standards, such as European Pharmacopoeia (202409-33-4 EP), 202409-33-4 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (202409-33-4 USP).
