Since 2022, layoffs have become commonplace. The ongoing global banking crisis, coupled with pre-existing factors such as the Ukraine-Russia conflict, inflation, looming recession and rising interest rates have made the business environment even more volatile and daunting. The kinks in the supply chain got exacerbated by China’s Covid policy. And all these economic challenges compelled companies to retrench employees the world over. While the surge in layoffs has been more apparent in the technology sector,
the pharma sector has also been facing the headwinds.Though the biopharma sector has
experienced significant growth due to new technologies such as gene editing,
cell therapy, messenger RNA and the Covid-19 vaccines and therapies,
some drugmakers began to see revenues of their Covid products fall
significantly with a drop in cases.However, job cuts in the pharma industry aren’t limited to companies that make Covid products. Several others have announced job cuts citing restructuring, trial failures and holds, termination of deals, facility shutdowns and reprioritization of projects.In the first quarter (Q1) of 2022, we saw
around 30 pharma companies announce layoffs. In Q1 of 2023, the corresponding
number went up to over 50. Overall, more than 100 biopharma companies had
announced layoffs in 2022.View Biopharma Layoff Tracker: 2022–Mid April '23 (Free Excel Available)Pandemic-hit Grifols to cut 2,300 jobs;
Catalent, Thermo Fisher lay off hundredsSpanish pharma Grifols has announced the biggest layoff of 2023 so far — it plans to axe 8.5 percent of its global workforce, or around 2,300 employees, to save €400 million (US$ 427 million) annually. The Covid-19 pandemic dealt a severe blow to its plasma-derived medicines as blood collection collapsed around the world in 2020 and 2021. The drugmaker is now working on a “more efficient” platform to obtain plasma and reduce its expenses.French vaccine maker Valneva said it will lay off 20 to 25 percent of its workforce in order to save US$ 12 million. Valneva cited clinical trial costs and expedited winding down of Covid vaccine-related activities as reasons behind the layoffs. Some CDMOs have been hit equally badly.
For instance, Catalent, which has played a critical
role in producing Covid-19 vaccines and therapies, has cut around 600 jobs across
multiple facilities in the US, and Thermo Fisher Scientific, a
producer of Covid testing kits, has laid off around 500 employees across various locations in California
between January 2022 and the middle of April 2023.Meanwhile, US-based Axcella said it will lay off 85 percent of its staff as it ended work on
its NASH program to focus on developing a long Covid therapy. Last July,
another US company, Inovio, said it will cut 18 percent of its workforce due to its troubled Covid program. And this year, it plans
to downsize again and focus on its human
papillomavirus (HPV) program.View Biopharma Layoff Tracker: 2022–Mid April '23 (Free Excel Available)Novartis to cut up to 8,000 jobs, J&J to downsize as part of restructuringBig pharma companies such as Novartis and Johnson & Johnson (J&J) have announced plans to restructure their businesses, and job cuts are a part of that exercise. In April 2022, Novartis announced plans to save at least US$ 1 billion by 2024 by combining its
pharmaceuticals and oncology business units to form a new Innovative Medicine
unit with the goal of achieving sales growth of
at least 4 percent through 2026. It also revealed plans to spin off Sandoz to focus on patented prescription medicines. As a result of these changes, the Swiss drugmaker announced plans to eliminate up to 8,000 jobs.Novartis is moving ahead with the job
cuts and has announced plant closures in the US. In addition, Novartis slashed 400 jobs in India following a new sales
and distribution agreement with Dr. Reddy’s Laboratories.Similarly, as J&J moves ahead with plans to spin off its consumer health, it is cutting jobs. J&J is also restructuring the infectious disease and vaccine groups of its Janssen division and has planned global layoffs. Fate Therapeutics, a former partner of J&J, laid off 315 staff after ending its agreement with
the company. Gilead has also cut jobs at
the former Immunomedics headquarters in New Jersey and
relocated the site to a larger space with no manufacturing.View Biopharma Layoff Tracker: 2022–Mid April '23 (Free Excel Available)Merck,
BMS cut staff at acquired companies; Biogen downsizes due to Aduhelm, TecfideraMany retrenchments in the biopharma
sector have been a result of acquisitions. Merck laid off around 143 employees from its Acceleron division in Cambridge,
Massachusetts, shortly after acquiring the Boston-based company for US$ 11.5
billion in March 2022.BMS has also laid off 261 employees across
two San Diego sites following its acquisition of Turning Point Therapeutics for
US$ 4.1 billion. Similarly, US biotech Flexion Therapeutics laid off 110 employees, after being acquired by Pacira BioSciences. Six months after acquiring Kadmon Holdings, Sanofi is closing its Kadmon New
York facility and laying off 25 employees. Sanofi-Aventis Korea is reducing its workforce
through a voluntary retirement scheme. And AbbVie laid off 99 staffers from a single facility in Irvine (California), which was once an Allergan facility (a company it acquired in 2019 for US$ 63 billion).In 2022, Amgen made some high-value acquisitions and agreements – it bought rare disease drugmaker, Horizon Therapeutics, for US$ 27.8 billion and ChemoCentryx for US$ 4 billion as part of its growth strategy.
But this year, Amgen has announced layoffs on two occasions, retrenching a total of around 750 employees to realign its expenses in the
face of intensifying pressure on drug prices and high inflation. And Roche’s Genentech unit has shut down operations at its production facility in South San Francisco, laying off 265.In December 2021, Biogen had announced plans to lay off
up to 1,000 staffers in an effort to cut about US$ 500-750 million in costs following the lower-than-expected sales of its controversial Alzheimer’s disease drug, Aduhelm, This year, Biogen has trimmed
its multiple sclerosis team due to generic competition to its blockbuster drug Tecfidera. The job cuts were necessitated after the company failed to defend the market exclusivity of the drug through several lawsuits in the US.Japanese drugmakers Daiichi Sankyo and Eisai have shut down their R&D units in the US. While Daiichi has closed its R&D unit in San Francisco that employed 60 people, Eisai has closed its oncology R&D wing in the US, H3 Biomedicine, a move that has resulted in the loss of 88 jobs.View Biopharma Layoff Tracker: 2022–Mid April '23 (Free Excel Available)Drug
rejections, trial failures lead to job cuts at Akebia, Y-mabs, SpectrumSeveral companies, such as Akebia, Y-mabs, Spectrum, have laid off sizable portions
of their workforce due to drug rejections by the US Food and Drug
Administration (FDA). Akebia laid off 42 percent of its workforce after FDA rejected its anemia drug vadadustat. Similarly, Spectrum laid off
most of its R&D team after FDA rejected its drug poziotinib in November 2022. In January
2022, it had terminated 30 percent of its
workforce. The same was the case with Y-mabs — a substantial number of employees lost their jobs after FDA rejected its drug omburtamab. In April 2022, Bluebird bio reduced its workforce by 30 percent to cut costs. Its gamble paid off as the FDA approved two of its cell and gene therapies – Skysona and Zynteglo – a few months later.Meanwhile, Galapagos dropped its kidney and fibrosis programs to focus on oncology and immunology candidates, laying off around 200 staffers. And disappointing data from a late-stage trial of its drug for symptomatic transthyretin amyloid cardiomyopathy (ATTR-CM) — avramidis — forced BridgeBio to announce retrenchment of an
undisclosed number of employees last year.View Biopharma Layoff Tracker: 2022–Mid April '23 (Free Excel Available)Our
viewThe biopharma industry grew impressively
in 2022. Our analysis of combined (global) revenues of 15 leading (randomly
selected) drugmakers in 2021 and 2022 reveals an impressive growth in revenues
of around 7 percent. However, in 2023, a third of these drugmakers expect a
drop in revenues, with Pfizer expecting its turnover to drop
by over 30 percent this year. The reduced guidance may result in more layoffs.As we move into Q2, the layoff trend
continues unabated. Last month, Thermo Fisher announced it will lay off 218 employees at
three of its locations in San Diego, California, due to reduced demand for its
Covid-19 products. These facilities will close in June. Japanese drugmaker Sumitomo and its subsidiary Sunovion Pharmaceuticals
have announced plans to lay off 223 workers.The current business environment is not likely to cheer the job market. We have to wait for the cycle to turn.
Impressions: 2202
Acquisitions and spin-offs dominated headlines in 2019 and the tone was set very early with Bristol-Myers Squibb acquiring
New Jersey-based cancer drug company Celgene in a US$ 74 billion deal announced on
January 3, 2019. After factoring
in debt, the deal value ballooned to about US$ 95 billion, which according
to data compiled by Refinitiv, made it the largest healthcare deal on
record.
In the summer, AbbVie Inc,
which sells the world’s best-selling drug Humira, announced its acquisition of Allergan Plc, known for Botox and other cosmetic
treatments, for US$ 63 billion. While the companies are still awaiting
regulatory approval for their deal, with US$ 49 billion in combined 2019
revenues, the merged entity would rank amongst the biggest in the industry.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
The big five by pharmaceutical sales — Pfizer,
Roche, J&J, Novartis and Merck
Pfizer
continued
to lead companies by pharmaceutical sales by reporting annual 2019 revenues of
US$ 51.8 billion, a decrease of US$ 1.9 billion, or 4 percent, compared to
2018. The decline was primarily attributed to the loss of exclusivity of Lyrica in 2019,
which witnessed its sales drop from US$ 5 billion in 2018 to US$ 3.3 billion in
2019.
In 2018, Pfizer’s then incoming CEO Albert Bourla had mentioned that the company did not see the need for any large-scale M&A activity as Pfizer had “the best pipeline” in its history, which needed the company to focus on deploying its capital to keep its pipeline flowing and execute on its drug launches.
Bourla stayed true to his word and barring the acquisition of Array Biopharma for US$ 11.4 billion and a spin-off to merge Upjohn, Pfizer’s off-patent branded and generic established medicines business with
Mylan, there weren’t any other big ticket deals which were announced.
The
Upjohn-Mylan merged entity will be called Viatris and is expected to have 2020
revenues between US$ 19 and US$ 20 billion
and could outpace Teva to
become the largest generic company in the world, in term of revenues.
Novartis, which had
followed Pfizer with the second largest revenues in the pharmaceutical industry
in 2018, reported its first full year earnings after spinning off its Alcon eye
care devices business division that
had US$ 7.15 billion in 2018 sales.
In 2019,
Novartis slipped two spots in the ranking after reporting total sales of US$
47.4 billion and its CEO Vas Narasimhan continued his deal-making spree by buying New
Jersey-headquartered The Medicines Company (MedCo) for US$ 9.7
billion to acquire a late-stage cholesterol-lowering
therapy named inclisiran.
As Takeda Pharmaceutical Co was
busy in 2019 on working to reduce its debt burden incurred due to its US$ 62
billion purchase of Shire Plc, which was announced in 2018, Novartis also purchased
the eye-disease medicine, Xiidra, from the Japanese drugmaker for US$ 5.3 billion.
Novartis’ management also spent a considerable part of 2019 dealing with data-integrity concerns which emerged from its 2018 buyout of AveXis, the
gene-therapy maker Novartis had acquired for US$ 8.7 billion.
The deal gave Novartis rights to Zolgensma,
a novel treatment intended for children less than two years of age with the
most severe form of spinal muscular atrophy (SMA). Priced at US$ 2.1 million,
Zolgensma is currently the world’s most expensive drug.
However,
in a shocking announcement, a month after approving the drug, the US Food and
Drug Administration (FDA) issued a press release on
data accuracy issues as the agency was informed by AveXis that
its personnel had manipulated data which
the FDA used to evaluate product comparability and nonclinical (animal)
pharmacology as part of the biologics license application (BLA), which was
submitted and reviewed by the FDA.
With US$
50.0 billion (CHF 48.5 billion) in annual pharmaceutical sales, Swiss drugmaker
Roche came in at number two position in 2019
as its sales grew 11 percent driven by
its multiple sclerosis medicine Ocrevus, haemophilia drug Hemlibra and cancer medicines Tecentriq and Perjeta.
Roche’s newly introduced medicines generated US$ 5.53 billion (CHF 5.4 billion) in growth, helping offset the impact of the competition from biosimilars for its three best-selling drugs MabThera/Rituxan, Herceptin and Avastin.
In late 2019, after months of increased
antitrust scrutiny, Roche completed
its US$ 5.1 billion acquisition of Spark Therapeutics to strengthen its presence in
gene therapy.
Last year, J&J reported almost flat worldwide sales of US$ 82.1 billion. J&J’s pharmaceutical division generated US$ 42.20 billion and its medical devices and consumer health divisions brought in US$ 25.96 billion and US$ 13.89 billion respectively.
Since J&J’s consumer health division sells analgesics, digestive health along with beauty and oral care products, the US$ 5.43 billion in consumer health sales from over-the-counter drugs and women’s health products was only used in our assessment of J&J’s total pharmaceutical revenues. With combined pharmaceutical sales of US$ 47.63 billion, J&J made it to number three on our list.
While the sales of products like Stelara, Darzalex, Imbruvica, Invega Sustenna drove J&J’s pharmaceutical business to grow by 4 percent over 2018, the firm had to contend with generic competition against key revenue contributors Remicade and Zytiga.
US-headquartered Merck, which is known as
MSD (short for Merck Sharp & Dohme) outside the United States and
Canada, is set to significantly move up the rankings next year fueled by its
cancer drug Keytruda, which witnessed a 55
percent increase in sales to US$ 11.1 billion.
Merck reported total revenues of US$ 41.75 billion and also
announced it will spin off its women’s health drugs,
biosimilar drugs and older products to create a new pharmaceutical
company with US$ 6.5 billion in annual revenues.
The firm had anticipated 2020 sales between US$ 48.8 billion and US$ 50.3 billion however this week it announced that the coronavirus pandemic will reduce 2020 sales by more than $2 billion.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Humira holds on to remain world’s best-selling drug
AbbVie’s acquisition of Allergan comes as the firm faces the expiration of patent protection for Humira, which brought in a staggering US$ 19.2 billion in sales last year for
the company. AbbVie has failed to successfully acquire or develop a major new
product to replace the sales generated by its flagship drug.
In 2019, Humira’s US revenues increased 8.6 percent to US$ 14.86 billion while internationally, due
to biosimilar competition, the sales dropped 31.1 percent to US$ 4.30 billion.
Bristol Myers Squibb’s Eliquis, which is also marketed by Pfizer, maintained its number two position
and posted total sales of US$ 12.1 billion, a 23 percent increase over 2018.
While Bristol Myers Squibb’s immunotherapy treatment Opdivo, sold in partnership with Ono in Japan, saw sales increase from US$ 7.57 billion to US$ 8.0 billion, the growth paled in comparison to the US$ 3.9
billion revenue increase of Opdivo’s key immunotherapy competitor Merck’s Keytruda.
Keytruda took the number three spot in drug sales that
previously belonged to Celgene’s Revlimid, which witnessed a sales decline from US$ 9.69 billion to US$ 9.4 billion.
Cancer treatment Imbruvica, which is marketed
by J&J and AbbVie, witnessed a 30 percent increase in sales. With US$ 8.1
billion in 2019 revenues, it took the number five position.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Vaccines – Covid-19 turns competitors into partners
This year has been dominated by the single biggest health emergency in years — the novel coronavirus (Covid-19) pandemic. As drugs continue to fail to meet expectations, vaccine development has received a lot of attention.
GSK reported the highest vaccine sales of all drugmakers with
total sales of US$ 8.4 billion (GBP 7.16 billion), a significant portion of its
total sales of US$ 41.8 billion (GBP 33.754 billion).
US-based Merck’s vaccine division also reported a significant increase in sales to US$ 8.0 billion and in 2019 received FDA and EU approval to market its Ebola vaccine Ervebo.
This is the first FDA-authorized vaccine against the deadly virus which causes
hemorrhagic fever and spreads from person to person through direct contact with
body fluids.
Pfizer and Sanofi also reported an increase in their vaccine sales to US$ 6.4
billion and US$ 6.2 billion respectively and the Covid-19 pandemic has recently
pushed drugmakers to move faster than ever before and has also converted
competitors into partners.
In a rare move, drug behemoths — Sanofi and GlaxoSmithKline (GSK) —joined hands to develop a vaccine for the novel coronavirus.
The two companies plan to start human trials
in the second half of this year, and if things go right, they will file
for potential approvals by the second half of 2021.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Our view
Covid-19 has brought the world economy to a grinding halt and shifted the global attention to the pharmaceutical industry’s capability to deliver solutions to address this pandemic.
Our compilation shows that vaccines and drugs
for infectious diseases currently form a tiny fraction of the total sales of
pharmaceutical companies and few drugs against infectious diseases rank high on
the sales list.
This could well explain the limited range of
options currently available to fight Covid-19. With the pandemic currently infecting
over 3 million people spread across more than 200 countries, we can safely
conclude that the scenario in 2020 will change substantially. And so should our
compilation of top drugs for the year.
View Our Interactive Dashboard on Top drugs by sales in 2019 (Free Excel Available)
Impressions: 54754
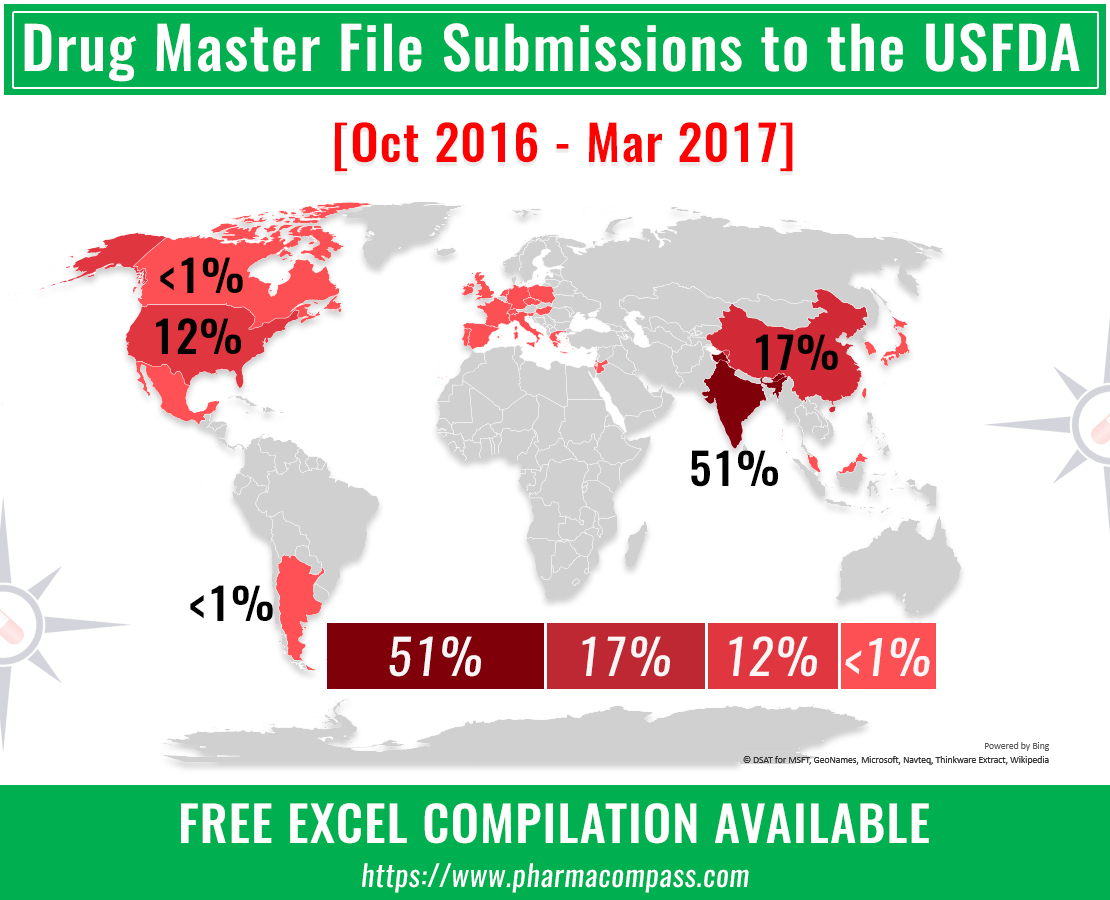
A review of the Drug Master Files (DMFs) submitted to the United
States Food and Drug Administration (FDA) from October 2016 to March 2017 (the
fourth quarter of 2016 and the first quarter of 2017) indicates an extremely
robust pharmaceutical industry in India. However, the filing make one question
an article we had carried earlier this month on the end of India’s pharma honeymoon.
India filed more than half the DMFs submitted
Indian companies filed more than half (176) of the 345 DMFs
submitted with the FDA. China (60) came a distant second, followed by the
United States (40). While DMF submissions were made from 26 other countries,
the activity levels seen were a far cry from what was seen in India and
China.
In our past compilation for the second and third quarters of 2016,
Macleods Pharmaceuticals (14 DMFs) had pipped MSN Laboratories (13 DMFs) to become the leading filer from
India.
However, in the recent review period, MSN bounced back with 41 DMF
submissions, compared to six for Macleod. Leading Indian pharmaceutical majors
such as Aurobindo,
Sun Pharma, Amneal,
Mylan’s
India operations, Hetero,
Cipla
and Jubilant
had six or more filings each.
Ajinomoto’s
North American operations filed 11 DMFs for various amino acids and led the
submissions for products manufactured in the United States.
Tianjin Weijie Pharmaceutical led the pack from China, with eight DMF
submissions.
Over the review period, a total of 345 submissions were made to the
FDA, almost similar to the 379 DMFs filed during the second and third quarters
of 2016.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
The next FTF challenges
Since APIs form the building blocks of finished formulations, DMF
submissions give a sneak preview into the next possible first-to-file (FTF)
generic challenges to patented drugs.
In
December 2016, Indian drug major Sun Pharmaceutical Industries
announced it will acquire a branded oncology product, Odomzo,
from Novartis
for an upfront payment of US$ 175 million.
A little over three months after the announcement, MSN Labs filed the first DMF for Odomzo’s active pharmaceutical ingredient (API), sonidegib phosphate. Odomzo was approved by the FDA in July
2015 and is indicated for the treatment of adult patients with locally advanced
basal cell carcinoma.
While patents for Novartis’ drugs Tafinlar
and Mekinist
are not scheduled to expire until 2029, Novartis should prepare for a generic
competitor as DMFs were filed for the APIs used in both drugs by MSN Labs. The
drugs had combined sales in 2016 of US$ 672 million.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
In addition, MSN Labs also filed the first DMF for nintedanib esylate, the API used in Boehringer Ingelheim’s idiopathic pulmonary fibrosis treatment,
Ofev, and for cabozatinib (S)-malate, the API used by Exelixis for its kidney cancer treatment — Cabometyx.
While in most cases MSN’s DMF is the first one to get filed, in the case of Helsinn’s
Akynzeo,
which is used to prevent chemotherapy-induced nausea and vomiting, Apicore US also filed a DMF along with MSN.
Although sales forecasts for Bayer’s pulmonary arterial hypertension treatment — Adempas (riociguat) — were lowered as the drug did not get the expected sales start
and planned label expansions did not materialize, this did not stop MSN Labs
from filing the first DMF for this product as well.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
Another drug which is struggling to meet analyst expectations is Novartis’ Entresto. Once considered “one of the most important products in the company's history” with an expectation to reach US$ 10 billion in peak sales, the drug generated sales of only US$ 170 million in 2016. Regardless, Mylan filed the first DMF for the API.
Another drug where MSN Labs did not file the first DMF was for AstraZeneca’s
ovarian cancer treatment, Lynparza (olaparib).
Alp Pharm Beijing submitted the DMF for the drug which
generated US$ 218 million in sales in 2016 and a figure Bernstein Research
analyst Timothy Anderson forecasts will grow to US$ 684 million by 2020.
The onslaught on Novartis’ portfolio is not limited to only patented products as Lamprene,
a product which has been on the market since 1986, and not had any generic
competitor, had Zhejiang Huahai file a DMF indicating a generic competitor maybe on the horizon
soon.
Mylan’s Sotradecol
has been the only injectable form of sodium tetradecyl sulfate
on the market since 2004. That monopoly may end soon as the FDA completed the
review of a DMF filed for the API early this year.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
Most actively filed products — vortioxetine hydrobromide and dolutegravir sodium
The most actively filed DMFs, with seven filings each, were those
for Takeda’s
depression drug Trintellix (vortioxetine hydrobromide) and GSK’s HIV treatment dolutegravir sodium.
Johnson & Johnson’s diabetes treatment Invokana (canagliflozin), which recently demonstrated that it
decreased the risk of heart attacks and strokes, while increasing the risk of amputation,
particularly of toes, had six new DMFs filed.
There were also six new DMFs filed for apremilast,
five for darunavir
and four each for dapagliflozin
and rivaroxaban.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
Filings for new drugs under development
Helsinn is moving ahead with the filing of anamorelin hydrochloride, as it filed DMFs for the API and the 100
mg tablets, a product which was welcomed enthusiastically by oncology experts as it raised hopes to
be a drug for cancer cachexia, the extreme wasting seen at the end stages of
the disease.
But those hopes were recently dashed, as a review of the clinical
data by the European Medicines Agency's Committee for Medicinal Products for
Human Use (CHMP) found only "marginal" effects and recommended that
the product be refused marketing authorization in Europe.
Now it remains to be seen what the FDA’s verdict on this drug will be.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
AB Science’s Masitinib has been in the news recently as an EMA committee announced the drug, developed for a range of cancers, could not be approved due to “serious failings” in the way clinical studies were conducted.
However, this did not stop Excella GmbH from filing its second DMF for the API.
Multiple sclerosis treatment dimethyl fumarate (Biogen’s Tecfidera) generated
sales of US$ 3.97 billion in 2016 and is projected to achieve US$ 5.56 billion by 2020.
While there are now 28 DMFs filed for dimethyl fumarate, in March
this year Alkermes
announced the initiation of a new phase 3 study
of ALKS 8700, a novel, oral monomethyl fumarate (MMF) prodrug candidate in
development for the treatment of relapsing forms of multiple sclerosis.
It remains to be seen when Alkermes’ product will get approved. However, MSN Labs followed Honour Labs to file the second DMF for this product.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
A new submission for deslorelin acetate
(an injectable gonadotropin releasing hormone super-agonist) indicates there
maybe a new drug development underway for this age-old peptide as currently
there are no approved drugs in the US.
A similar situation seems to exist for taurolidine,
an antimicrobial that seeks to prevent infections in catheters.
Vasudha Pharma’s filing of cisapride monohydrate comes as a surprise. The product, which
was launched by Janssen for increased motility of the
gastrointestinal tract, was later withdrawn from the US market due to concerns of
fatalities linked to cardiac arrhythmias.
The product, however, continues to be exported from India to
countries like Switzerland, Thailand, Mexico, China and Canada.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
Our view
The last quarter of 2016 and the first quarter of 2017 clearly
demonstrate an API industry in India and China, which is extremely active with
new product development, regardless of disappointing financial results posted
by major pharma companies and growing concerns over regulatory non-compliances.
Given the market headwinds and increased compliance expectations,
it remains to be seen how many of these DMFs filed actually result in drugs
reaching the market.
Click here to view all the DMF submissions in Q4 2016 and Q1 2017 (Excel version available) for FREE!
Impressions: 5931
This week, PharmaCompass brings you a compilation of the Drug Master Files (DMFs) updates at the US Food and Drug Administration (FDA) over the past two quarters. These applications provide an overview of the products active pharmaceutical ingredient (API) manufacturers are investing in. And, they also give a sneak preview into the next possible first-to-file (FTF) generic challenges to patented drugs.
Here are some key findings from our compilation of the FDA’s DMF updates over the second and third quarter of 2016, details of which were provided in July and October:
India leads
the pack, as the number of filings remain the same
Over the period, there were a total of 379 updates of DMFs at the FDA. This number
indicates a pace in filings that is nearly the same as the previous quarters.
We had seen 180 DMFs updates in the last quarter (Q4) of 2015 and 190 in the first quarter (Q1) of 2016.
During the last
two quarters, the DMF updates were led by Indian companies, such as Macleods Pharmaceuticals (14 DMFs), MSN Labs (13 DMFs), Hetero
(12 DMFs), Lupin (9 DMFs), Cipla and Biophore. The other prominent companies were Mylan and Teva.
Companies
with compliance issues stay away
Unlike previous
quarters, where
companies with compliance problems continued to submit DMFs, the last two
quarters were slightly different, since companies like Zhejiang Hisun and Ipca
Laboratories did not submit DMFs.
However,
Emcure Pharmaceuticals — whose Pune facility was inspected by
the FDA last year and a warning letter was issued to the company for violations of current good manufacturing practices (cGMPs) in March this year — submitted one DMF (for Phytonadione)
While
China shut down antibiotic manufacturing in the Shijiazhuang
city, raising concerns about the global supply chain’s dependence on China, Sinopharm Weiqida Datong
Pharmaceutical, located about 300 kilometers away from Shijiazhuang, filed DMFs for the key building blocks of antibiotic manufacturing — 6-APA and 7-ACA.
Once
again, this filing reinforces the dependence of global
antibiotic manufacturing on China.
Click here to view all the updates of the second and third quarter of 2016 (Excel version available) for FREE!
Imminent FTF challenges
The
FTF challenges to Alvimopan Dihydrate (Merck’s Entereg), Apremilast (Celgene’s Otezla), Bosutinib (Pfizer’s Bosulib), Daclatasvir
Dihydrochloride (Bristol-Myer
Squibb’s Daklinza), Elvitegravir (an ingredient in Gilead’s Vitekta,
Stribild, Genvoya), Ibrutinib (AbbVie’s
Imbruvica), Ospemifene (Shionogi’s Osphena), Perampanel (Eisai’s Fycompa), Pomalidomide (Celgene’s Pomalyst), Regorafenib (Bayer’s Stivarga), Tofacitinib (Pfizer’s Xeljanz) and
Vortioxetine
Hydrobromide (Takeda’s Trintellix) seem to be imminent in
view of the recent filings of DMFs.
Roche’s 2014 acquisition of
InterMune for US $ 8.3 billion to gain rights to Esbriet (pirfenidone)
is likely to come under attack as three more DMFs were submitted during the
period under review.
The ink wasn’t dry on the deal papers of Pfizer’s US $1 4 billion acquisition of Medivation in August this year, when two more companies — Watson Pharma (now Allergan) and Scinopharm — submitted filings for Enzalutamide, the product for
which Pfizer paid all that money. This takes the total number of US submissions
for this product to seven.
Apixaban and Canagliflozin are most actively filed products
The most actively updated DMFs in the past six months were for the APIs of Bristol-Myer Squibb’s new-age anticoagulant Eliquis (Apixaban)
and Johnson & Johnson’s diabetes treatment Invokana (Canagliflozin). Sixteen DMFs were submitted for Apixaban along with nine for
Canagliflozin.
Products
like Dimethyl Fumarate and Teriflunomide — which were the
most frequently filed DMFs in our previous reports — continued to see vigorous filing activity.
Synbias Pharma made a submission
for Nelarabine, the only
submission for a Novartis product that was approved in 2005
and for which the only listed patent is expiring in June 2017. Similarly DSM’s submission of Dexpanthenol is the only DMF
listed for a product used in a variety of injectable and intravenous solution
products.
Established
pharmaceutical companies like Quimica Sintetica and Piramal Healthcare made submissions for products — Benznidazole and Norprostol — which are currently not approved in the United States, indicating the possibility of development projects being underway.
Our view
With drug filings ranging from multiple FTFs to cannabis derivatives,
updates over the two quarters have shown that regardless of the compliance
news, activity in the API industry is extremely robust.
You can view the PharmaCompass compilation of
the new DMF filings by clicking here or simply by sending us an email to get
your own Excel version of the new submissions.
Click here to view all the updates of the second and third quarter of 2016 (Excel version available) for FREE!
Impressions: 4466
Every quarter, PharmaCompass compiles the latest Drug Master Files (DMFs) submitted to the US Food and Drug Administration (FDA). These applications provide an overview of products which active pharmaceutical ingredient (API) manufacturers are investing their resources in and also give a sneak preview into the next possible first-to-file (FoF) generic challenges to patented drugs. Here are the key findings from the compilation for the first
quarter of 2016: Compliance problems
aside, India tops the DMF submissionsIf news about compliance problems faced by pharma companies
in India and China are making you believe that there is a slowdown in these
countries, think again. A total of 190 DMF submissions were made in the first
quarter of 2016, up from 180 in the previous quarter. And over two-thirds of
the submissions were for products from facilities based in either India or
China with more than 100 filings from India alone. Companies that have been on the compliance radar recently – such as Ipca
Laboratories, Emcure
Pharmaceuticals, Minsheng
Group Shaoxing Pharmaceutical and Yincheng Goto – also made submissions to the FDA. Besides these, Qilu
Pharmaceutical, which was in the news recently for the controversy in China
involving school children, also submitted its DMFs this quarter. Teriflunomide leads DMF
race; Carfilzomib is the new molecule on the blockLast quarter, Teriflunomide saw the maximum number of DMF submissions – four. This quarter too, Teriflunomide led the pack with maximum submissions – six. This is an indication that Sanofi’s
multiple sclerosis drug will be subject to severe competition in the coming
future.Amgen acquired Onyx
Pharmaceuticals for US $ 10.4 billion in 2013 primarily to cash in on the potential of Kyprolis
(carfilzomib), a cancer-treatment drug. While analysts had estimated peak sales of US $ 1.6 billion as a result of this acquisition, sales in 2014 turned out to be only US $ 331 million – a fifth of their estimates. However, things looked up in 2015 as Kyprolis brought in US $ 512 million in sales. Amgen needs to quickly capitalize on the opportunity as six more DMFs were submitted this quarter, indicating a severe generic onslaught whenever the drug goes off patent. Enzalutamide has been in news recently as Medivation, the US
cancer drug company that discovered the molecule, has finally become open to a
sell-off after Sanofi offered US $ 9.3 billion to buy the cancer drug
maker. Even as Sanofi tries to acquire Medivation, generic activity is underway
with three more DMF submissions this quarter. MSN Labs leads the FoF
challenges and filingsMSN Laboratories may not be well-known in the Indian pharmaceutical industry, but the company is growing from strength to strength each quarter with its capabilities of developing non-infringing routes for APIs and being one of the first companies to submit DMFs.This quarter, MSN
Laboratories and its subsidiaries submitted 20 DMFs, which is more than 10 percent of all applications filed. MSN’s filings include Apremilast
(Celgene’s Otezla), Bosutinib
(Pfizer’s
Bosulif), Macitentan
(Actelion’s
Opsumit) and Vortioxetine
Hydrobromide (Takeda’s
Brintellix) Innovative filings In
the worksBiotin, a water soluble Vitamin B, is claimed to aid nail and hair growth. If French biotech startup -- Medday Pharmaceuticals – succeeds in its Phase III trials, its lead product MD1003, a pharmaceutical grade
D-Biotin, would improve the lives of patients suffering from progressive
multiple sclerosis (MS).MedDay and DSM Nutritional Products had earlier announced a
partnership and co-investment for manufacturing pharmaceutical grade D-Biotin.
This quarter DSM submitted its DMF for Biotin.Switzerland-based biopharmaceutical company Debiopharm’s
Salvacyl®, Moapar® has been used mainly for the treatment of prostate cancer. Now, armed with a new indication, the three-month formulation of triptorelin – which has been registered in several European countries to treat severe sexual deviation in adult men (for instance, paedophilia) – saw a DMF submission this quarter indicating a potential launch of this product in the US.Dimethyl fumarate (Biogen’s Tecfidera) led
the list of DMF filings last quarter as the brand product generated sales of US
$ 3.64 billion in 2015 and is projected to achieve US $
5.56 billion by 2020. While generic companies have been targeting Dimethyl Fumarate, Hyderabad-headquartered
Honour Labs – a company promoted by Dr B Parthasaradhi Reddy
who is also the promoter of generic major Hetero Drugs – filed a DMF for Monomethyl Fumarate. It would be interesting to see if this minor tweaking in the
molecular structure could lead to a windfall gain for Hetero. Our viewWith drug filings ranging from cannabis extract to amphetamines
to generic paracetamol,
the first quarter of 2016 displayed that the API industry is extremely active
with new product development. You can view the PharmaCompass
compilation of the new DMF filings by clicking here or simply by sending us an
email to get your own Excel version of the new submissions.
Click here to view all the submissions of the first quarter of 2016 (Excel version available) for FREE!
Impressions: 5248
The year 2015 has gone down
in history as a record year for mergers and acquisitions in the pharmaceutical
and biotech space with deals worth US $ 300 billion being announced. The highlight
of the year was the Pfizer-Allergan mega-merger – the biggest-ever pharma transaction worth more than US $ 160 billion.
Pharma Letter tracked transactions
through the year and found the number of deals exceeding US $1 billion at 30 in
2015, as compared to 26 in 2014 and 20 in 2013. In all, a total of 166 M&A
deals were announced in 2015 (out of which some are yet to be completed),
compared to 137 in 2014.
This week, PharmaCompass
brings you a compilation of the top drugs of 2015 by sales revenue and growth.
Sofosbuvir – the outright winner of 2015
2015 was the year of Sofosbuvir – the revolutionary active ingredient used for the treatment of hepatitis. Together, through the sale of drugs Harvoni and
Sovaldi, Sofosbuvir brought in sales of almost US $ 19 billion.
The PharmaCompass prediction
that Harvoni (a combination of Ledipasvir and Sofosbuvir; and used for the treatment
of infectious diseases like hepatitis and HIV) would become the best-selling
drug ever in 2015 fell slightly short of expectations as its sales of US $ 13.864
billion were marginally less than AbbVie’s rheumatoid arthritis treatment – Humira.
Humira retained its place as the best-selling drug with US $
14.012 billion in sales in 2015. However, with sales growth of US $ 11.737
billion in a single year, Harvoni is poised to become the best-selling drug by
the end of 2016.
Top 20 Drugs by Sales
Here is PharmaCompass’ compilation of the best-selling drugs of 2015. This is based on information
extracted from annual reports and US Securities and Exchange Commission (SEC) filings
of major pharmaceutical companies.
If you would like your own copy of all the information we’ve collected, email us at support@pharmacompass.com and we’ll send you an Excel version.
Click here to access all
the 2015 data (Excel version available) for FREE!
Product
Active Ingredient
Main Therapeutic Indication
Company
2014 Revenue in Millions
(USD)
2015 Revenue in Millions
(USD)
2015 Sales Difference
Millions (USD)
1
Humira
Adalimumab
Immunology (Organ Transplant, Arthritis etc.)
AbbVie
12,543
14,012
1,469
2
Harvoni
Ledipasvir
and Sofosbuvir
Infectious Diseases (HIV, Hepatitis etc.)
Gilead
Sciences
2,127
13,864
11,737
3
Enbrel
Etanercept
Immunology (Organ Transplant, Arthritis etc.)
Amgen / Pfizer
4,688
8,697
4009
4
Remicade
Infliximab
Immunology (Organ Transplant, Arthritis etc.)
Johnson
& Johnson / Merck
6,868
8,355
1487
5
MabThera/Rituxan
Rituximab
Oncology
Roche
5,659
7,115
1,456
6
Lantus
Insulin Glargine
Diabetes
Sanofi
6,978
7,029
51
7
Avastin
Bevacizumab
Oncology
Roche
6,481
6,751
270
8
Herceptin
Trastuzumab
Oncology
Roche
6,338
6,603
265
9
Revlimid
Lenalidomide
Blood Related Disorders
Celgene
Corpoartion
4,980
5,801
821
10
Sovaldi
Sofosbuvir
Infectious Diseases (HIV, Hepatitis etc.)
Gilead
Sciences
10,283
5,276
(5,007)
11
Seretide / Advair
Salmeterol
Respiratory Disorders
GlaxoSmithKline
6,005
5,227
(778)
12
Crestor
Rosuvastatin
Calcium
Cardiovascular
AstraZeneca
5,512
5,017
(495)
13
Lyrica
Pregabalin
Neuroscience and Mental Health
Pfizer
Inc.
5,168
4,839
(329)
14
Neulasta
Pegfilgrastim
Blood Related Disorders
Amgen
4,596
4,715
119
15
Gleevec / Glivec
Imatinib
Oncology
Novartis
4,746
4,658
(88)
16
Xarelto
Rivaroxaban
Anticoagulants
Bayer / Johnson
& Johnson
3,369
4,345
976
17
Copaxone
Glatiramer
Neuroscience and Mental Health
Teva
4,237
4,023
(214)
18
Januvia
Sitagliptin
Diabetes
Merck
& Co
3,931
3,863
(68)
19
Abilify
Aripiprazole
Neuroscience and Mental Health
Bristol-Myers
Squibb/ Otsuka
Holdings
6,485
3,804
(2681)
20
Tecfidera
Dimethyl
Fumarate
Neuroscience and Mental Health
Biogen
2,909
3,638
729
Click here to access all
the 2015 data (Excel version available) for FREE!
A year of record FDA approvals
2015 was also the
year when the US Food and Drug Administration (FDA) approved 45 novel drugs, another
all-time record high. In January this year, PharmaCompass had compiled a list of novel drugs approved by the FDA in 2015. We also extensively covered the new dosage forms of existing drugs approved in 2015. Do go through the article published on January 14, 2016, for more information.
PharmaCompass’ compilation of sales forecasts of novel drugs indicated a significant
variation in estimates. However, in our view, drugs that
saw highest sales growth in 2015 are likely to do well this year as well.
Top 20 drugs by sales growth (in USD, millions)
Product
Active Ingredient
Main Therapeutic Indication
2014 Revenue in Millions
(USD)
2015 Revenue in Millions
(USD)
2015 Sales Difference
Millions (USD)
1
Harvoni
Ledipasvir
and Sofosbuvir
Infectious Diseases (HIV, Hepatitis etc.)
2,127
13,864
11,737
2
Viekira Pak
Ombitasvir/Paritaprevir/Ritonavir
Infectious Diseases (HIV, Hepatitis etc.)
48
1,639
1,591
3
Humira
Adalimumab
Immunology (Organ Transplant, Arthritis etc.)
12,543
14,012
1,469
4
Hepatits C Franchise
Daclatasvir and Asunaprevir
Infectious Diseases (HIV, Hepatitis etc.)
256
1,603
1,347
5
Imbruvica
Ibrutinib
Chronic lymphocytic leukemia
200
1,443
1,243
6
Cubicin
Daptomycin
Anti-bacterial
25
1,127
1,102
7
Eliquis
Apixaban
Anticoagulants
774
1,860
1,086
8
Triumeq
Abacavir, Dolutegravir and Lamivudine
Infectious Diseases (HIV, Hepatitis etc.)
-
1,037
1,037
9
Xarelto
Rivaroxaban
Anticoagulants
3,369
4,345
976
10
Opdivo
Nivolumab
Oncology
6
942
936
11
Revlimid
Lenalidomide
Blood Related Disorders
4,980
5,801
821
12
Tecfidera
Dimethyl
Fumarate
Neuroscience and Mental Health
2,909
3,638
729
13
Xtandi
Enzalutamide
Oncology
480
1,207
727
14
Ibrance
Palbociclib
Oncology
-
723
723
15
Invokana / Invokamet
Canagliflozin
Type 2 diabetes
586
1,308
722
16
Victoza
Liraglutide
Diabetes
2,014
2,704
690
17
Stribild
Cobicistat, Elvitegravir, Emtricitabine and Tenofovir
Disoproxil Fumarate
Infectious Diseases (HIV, Hepatitis etc.)
1,197
1,825
628
18
Levemir
Insulin
Diabetes
2,133
2,745
612
19
Votrient
Pazopanib
Oncology
565
565
20
Perjeta
Pertuzumab
Oncology
927
1459
532
Hepatitis C products, which had three
of the four highest sales growths in 2015, clearly show the impact these
revolutionary treatments will have on the global healthcare landscape in time
to come. Cancer immunotherapy treatments, a new generation of blood thinners
and novel diabetes treatments were some of the others which demonstrated stellar
growth in 2015.
Vaccines from Pfizer and Sanofi also displayed tremendous sales growth although they
have not been included in the compilation of drugs.
Click here to access all
the 2015 data (Excel version available) for FREE!
Sign Up, Stay Ahead
While some companies like Boehringer and Valeant are yet to release their annual reports. In order to
stay informed, do sign up for the PharmaCompass
Newsletter and you will receive updated information as it becomes available
along with a lot more industry analysis.
Click here to access all
the 2015 data (Excel version available) for FREE!
CORRECTION, April 12, 2016: An earlier version of this compilation
did not account for cases where the same drug is sold by multiple companies
(e.g. Enbrel, Remicade, Xarelto etc.). As an outcome, a re-ranking of the Top
20 Drugs by Sales and Sales Growth has been done.
Impressions: 56517
With almost 30,000 Drug Master Files (DMFs) submitted to the
FDA, reviewing the filings of only the first quarter of 2015, provides an
indicator on the current areas of focus of generic pharmaceutical companies. A
detailed evaluation of the 241 filings for active pharmaceutical ingredients only,
made us find some interesting trends worth sharing.
European Blockbuster
battle!
Of the 241 DMFs, 21 APIs had more than one DMF filing and
accounted for 25% of the total filings. Interestingly, 20 DMFs were for only three APIs:
AstraZeneca’s blood thinner Brilinta® (Ticagrelor), with 2014 sales of $476
million, already had DMF filings from Dr. Reddy’s, Mylan,
Polpharma and
Zhejiang Hisun at the end of last year. With a maximum of 9 new filings from players
like Teva,
Alembic, Lek and
others, AstraZeneca
should brace itself for some serious generic onslaught.
While the 9 filings for Ticagrelor were the most for any
single compound, not far behind is Bayer’s own blood thinner: Xarelto® (Rivaroxaban). With 7 submissions, the
focus of the generic companies is understandable as Rivaroxaban had sales in
excess of $3 billion and year-on-year growth in excess of 70%. However, patents
currently protect the product till 2020, so patience is needed before generics can
access this golden opportunity.
Interestingly, 4 filings for Linagliptin (Boehringer’s antidiabetic Tradjenta®) make it yet another European pharma giant lead the list of products being subjected to generic competition, and make us wonder why European blockbusters are preferred over others?
Exclusive but not
patented
There are products, which have no patent protection, but the
market is protected by FDA granted exclusivities (learn more on patents
and exclusivities from the FDA website).
An opportunity for generic companies to gain significant
market share of a multi-hundred million dollar market, without any litigation
risk or cost is something companies dream about.
As the time of exclusivity expiry nears, Clobazam, Tetrabenazine,
Hydroxyprogesterone Caproate, Deferiprone
and Trypan Blue
will all see increased generic activity as their Drug Master Files have been
submitted.
Fragmented Activity
More than 80% of the DMF submissions were made by companies
who filed only a single product. While the products varied from simple compounds
like Sodium Chloride to biologics like Plasmid DNA, over 140 companies filed DMFs in
the first quarter with almost 30 submitting a DMF for the first time.
An expanding list of suppliers who support DMFs increases
options for sourcing managers. However,
a fragmented supplier base limits the industrial scale companies can achieve and
raises concerns regarding how many can successfully sustain compliance standards
under increased regulatory scrutiny?
The Next Generic Wave
Blood thinners are an opportunity few generic companies wish to pass on. Boehringer’s (Dabigatran Etexilate), Bristol-Myers Squibb’s (Apixaban) and Bayer’s (Rivaroxaban) are novel compounds in this category which had combined sales in excess of $5 billion last year.
While Dagibatran saw a flurry of activity over the last two
years with almost 15 DMF filings, there were no additional filings this year.
On the other hand, Apixaban, which generated $774 million
for Bristol-Myers Squibb in 2014, has only one DMF filing at the moment and that too was done
over a year ago. The export data out of India, reviewed on PharmaCompass, for
Apixaban, indicates that product development is already complete so it is just
a matter of time before the filings begin.
Conclusion:
Product and supplier selection is a critical component of every generic company’s strategy. The PharmaCompass database is designed to assist professionals in business development, marketing and sourcing to take more informed decisions.
If you would like us to share our shortlist of 241 DMFs, we will be happy to send it to you by email (click here). You can also access our compilation of the 2014 annual reports of
major pharmaceutical companies to review the various products along with their
revenues (click here):
Table: Products with more than one DMF filing in Q1 2015
PRODUCT NAME
DMF FILINGS
TICAGRELOR
9
RIVAROXABAN
7
LINAGLIPTIN
4
APREPITANT
3
CINACALCET HYDROCHLORIDE
3
ATAZANAVIR SULFATE
2
ATORVASTATIN CALCIUM TRIHYDRATE
2
CLOBAZAM
2
CLOFARABINE
2
DEFERASIROX
2
DIMETHYL FUMARATE
2
EZETIMIBE
2
ICATIBANT ACETATE
2
LURASIDONE HYDROCHLORIDE
2
MELPHALAN HYDROCHLORIDE
2
OLANZAPINE
2
OLMESARTAN MEDOXOMIL USP
2
PRASUGREL HYDROCHLORIDE
2
RIVASTIGMINE USP
2
ROSUVASTATIN CALCIUM
2
SOLIFENACINE SUCCINATE
2
Impressions: 8782