This week, PharmaCompass
reviews the recently released data on prescription drugs paid for under the
Medicare Part D Prescription Drug Program in the United States in calendar year
2016.
But first, let’s understand what is Medicare.
Medicare is the federal health insurance program in the US. In 2017, it covered 58.4 million people — 49.5 million aged 65 and older, and 8.9 million disabled.
Prescription drug coverage under this
program was started in 2006, and is known as Medicare Part D.
As part of this
coverage, the Centers for Medicare & Medicaid Services (CMS) contracts insurance
companies and other private companies, known as plan sponsors, that offer
prescription drug plans to their beneficiaries with varying drug coverage and
cost-sharing requirements.
In
2017, the Congressional Budget Office (CBO) had estimated that spending on
Medicare Part D would reach US$ 94 billion, or about 16 percent of all Medicare
expenditures for the year.
Click here to access the compilation of Medicare Part D
Prescriber Summary Report
According
to the CBO, Medicare Part D is the most significant expansion of the Medicare
program since it was created by Congress in 1965.
With
more than 1.48 billion claims from beneficiaries enrolled under the Part D
prescription drug benefit program under its umbrella, our analysis of Medicare
Part D provides valuable insights into how elderly Americans use prescription
drugs.
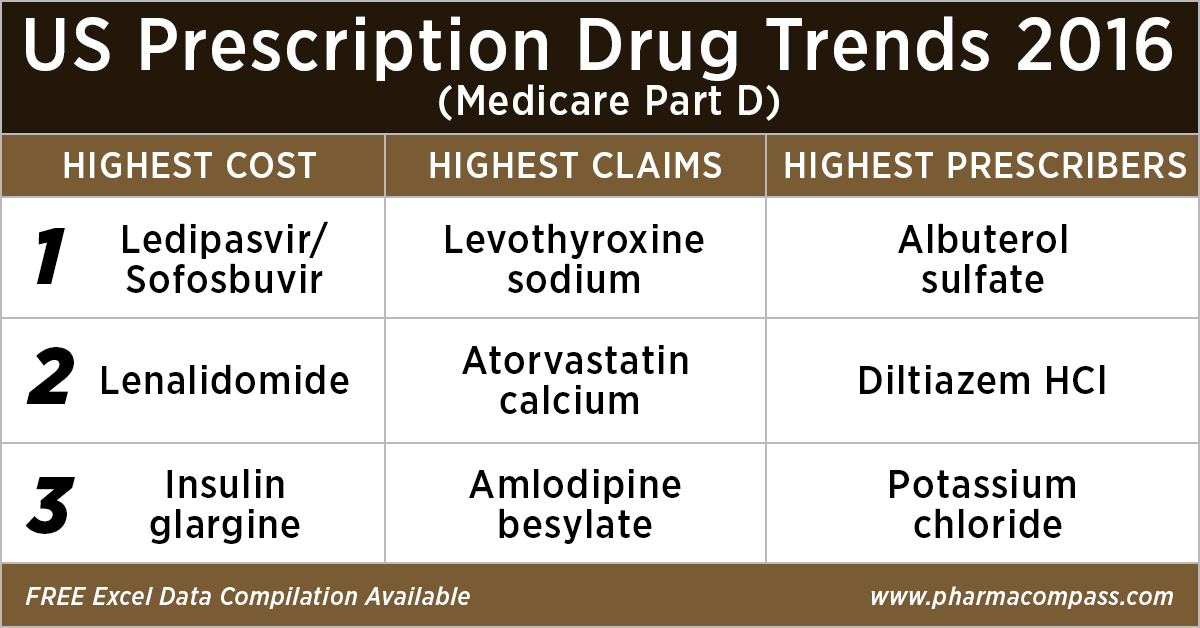
Top 10 drugs by
cost: The ones that bore the highest cost burden for Medicare
As in 2015, in 2016
too Gilead’s Hepatitis C treatment — Ledipasvir/Sofosbuvir (Harvoni) — remained the single drug highest payout under the Medicare Part D Prescription Drug Program with a total cost of US$ 4.4 billion.
As Gilead continued
to face competition from AbbVie and Merck in the Hepatitis C space, the spending on Harvoni was down
37 percent from US$ 7.03 billion in 2015.
Click here to access the compilation of Medicare Part D
Prescriber Summary Report
Celgene’s cancer treatment, Lenalidomide (Revlimid), Sanofi and Merck’s diabetes treatments and AstraZeneca’s Crestor (Rosuvastatin Calcium) for
cholesterol followed Harvoni. All together, they cost the Medicare program over US$ 10 billion.
Generic Name
Number of Medicare Part D Claims
Number of Medicare Beneficiaries
Number of Prescribers
Aggregate Cost Paid for Part D
Claims (In USD)
LEDIPASVIR/ SOFOSBUVIR (HARVONI)
141,665
52,782
12,097
4,398,534,465
LENALIDOMIDE
239,049
35,368
10,382
2,661,106,127
LANTUS SOLOSTAR (INSULIN
GLARGINE, HUM.REC.ANLOG )
5,028,485
1,075,248
245,447
2,526,048,766
SITAGLIPTIN PHOSPHATE
4,742,505
864,442
206,223
2,440,013,513
ROSUVASTATIN CALCIUM
6,012,444
1,560,050
249,981
2,322,724,007
FLUTICASONE/SALMETEROL
5,194,391
1,196,007
275,442
2,319,808,482
PREGABALIN
4,940,115
852,497
267,532
2,098,953,250
RIVAROXABAN
4,403,332
807,820
252,141
1,954,748,890
APIXABAN
4,455,782
826,969
231,631
1,926,107,484
TIOTROPIUM BROMIDE
4,153,162
903,494
235,564
1,818,857,361
Click here to access the compilation of Medicare Part D
Prescriber Summary Report
Top 10 drugs by claims: The most commonly
used drugs of 2016
With 46.6 million claims, the thyroid hormone deficiency treatment — Levothyroxine Sodium — retained its position of being the most claimed product under Medicare’s Part D Prescription Drug Program in 2016.
The number of
Medicare Part D claims includes original prescriptions and refills.
Following Levothyroxine Sodium was the lipid-lowering agent — Atorvastatin Calcium — which had 44.5 million Medicare Part D claims that
were filed by almost 9.4 million beneficiaries.
Generic
Name
Number
of Prescribers
Number
of Medicare Part D Claims
Number
of Medicare Beneficiaries
LEVOTHYROXINE SODIUM
669,999
46,617,109
8,091,785
ATORVASTATIN CALCIUM
494,973
44,595,686
9,435,633
AMLODIPINE BESYLATE
497,017
39,913,468
7,802,905
LISINOPRIL
490,452
39,469,840
8,009,954
OMEPRAZOLE
492,951
32,909,236
7,001,160
METFORMIN HCL
611,700
31,007,932
6,394,014
SIMVASTATIN
380,560
29,687,947
6,201,911
HYDROCODONE/ACETAMINOPHEN
660,617
28,595,150
7,265,882
FUROSEMIDE
488,352
27,878,243
5,421,598
GABAPENTIN
555,997
27,627,466
5,363,382
Click here
to access the compilation of Medicare Part D Prescriber Summary Report
Top 10 drugs by prescribers: Medicines that were most popular with
doctors
Among the prescribers, albuterol sulfate (salbutamol) and Diltiazem had
over 900,000 unique providers (or
doctors) prescribing the drug.
Albuterol (salbutamol) is
used to provide quick relief from wheezing and shortness
of breath while Diltiazem is used to prevent chest
pain (angina).
Also
on the list of popular drugs with prescribers is Hydrocodone-Acetaminophen.
With more doctors prescribing Hydrocodone-Acetaminophen (an
opioid) than commonly used antibiotics, such as Cephalexin, Ciprofloxacin and Amoxicillin, the
series of new FDA initiatives to combat the epidemic of opioid misuse and abuse
should change the position of opioids in the top 10 drugs by prescribers in the
coming years.
Click here to access the compilation of Medicare Part D
Prescriber Summary Report
Generic
Name
Number of
Prescribers
Number of
Medicare Part D Claims
Number of
Medicare Beneficiaries
ALBUTEROL SULFATE
985,427
13,100,354
5,417,718
DILTIAZEM HCL
931,159
8,142,004
1,982,550
POTASSIUM CHLORIDE
879,491
18,945,969
4,278,000
PEN NEEDLE, DIABETIC
677,210
5,281,778
1,795,046
LEVOTHYROXINE SODIUM
669,999
46,617,109
8,091,785
HYDROCODONE/ACETAMINOPHEN
660,617
28,595,150
7,265,882
METFORMIN HCL
611,700
31,007,932
6,394,014
CEPHALEXIN
597,647
5,603,879
3,933,373
CIPROFLOXACIN HCL
594,129
7,000,081
4,851,657
AZITHROMYCIN
591,028
7,958,625
5,734,122
What does the
future hold?
Although the Part D Prescriber PUF (public use file) has a wealth of information on payment and utilization for Medicare Part D prescriptions, the dataset has a number of limitations. Of particular importance is the fact that the data may not be representative of a physician’s entire practice or all of Medicare as it only includes information on beneficiaries enrolled in the Medicare Part D prescription drug program (i.e., approximately two-thirds of all Medicare beneficiaries).
Click here to access the compilation of Medicare Part D
Prescriber Summary Report
Last
month, the Office of the Inspector General (OIG)
reviewed
the Part D claims data for the years 2011 to 2015 for brand-name drugs.
The OIG’s report found that the total reimbursement for all brand-name drugs in Part D increased 77 percent from 2011 to 2015, despite a 17-percent decrease in the number of prescriptions for these drugs.
With soaring drug prices being an issue for
regular debate in the Unites States and President Trump announcing that his
team will use strategies to strengthen the negotiating powers under
Medicare Part D and Part B, it remains to be seen how the data on prescription drugs paid for under
the Medicare Part D Prescription Drug Program will change in the coming years.
Click here to access the compilation of Medicare Part D
Prescriber Summary Report
Impressions: 2500
The year 2017 was a landmark year for pharmaceutical
industries in the US and Europe, with a sharp increase in the number of new molecular entities (NMEs) being approved in both geographies.
The US Food
and Drug Administration (USFDA) approved 46 NMEs in 2017, the second highest
since 1996 when 53 NMEs were approved. In Europe, the European Medicines Agency
(EMA) approved 35 drugs with a new active substance, up from 27 in 2016.
Sales for most major pharmaceutical
companies continued to grow in 2017. Earnings forecasts for 2018 have been raised due to the recent US tax reform that has
generated investor hopes for accelerated dividend growth and share buyback
plans.
This week, PharmaCompass brings
you a compilation of the top drugs of 2017 by sales revenue.
Click here to Access All the 2017 Data (Excel
version available) for FREE!
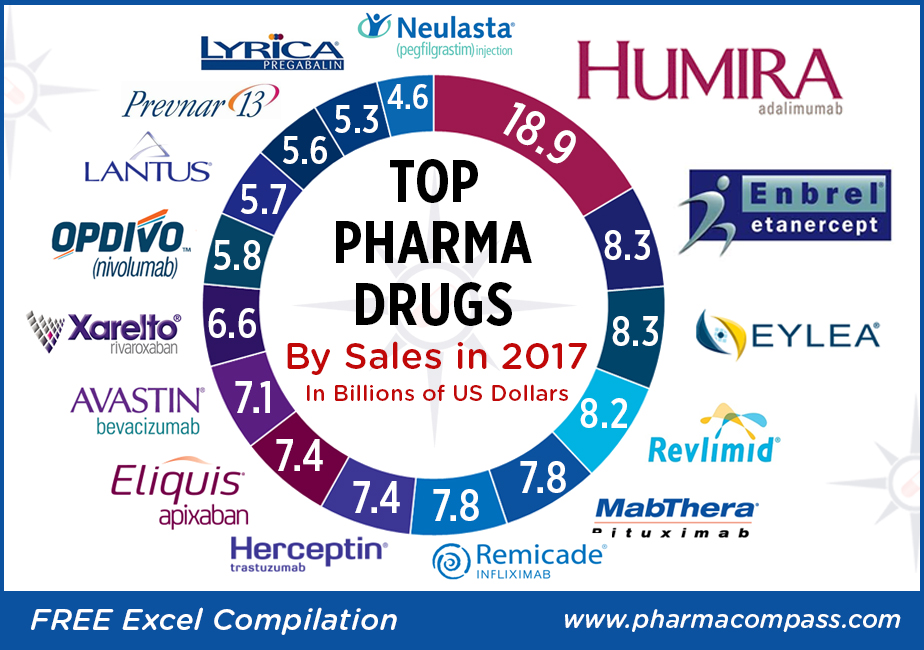
Top-sellers: Humira races ahead, despite launch of biosimilars; Enbrel a distant second
There wasn’t any upheaval
at the top of the pharma drug sales charts. AbbVie’s anti-TNF (tumor necrosis factor) giant
Humira (adalimumab), which is approved to treat
psoriasis and rheumatoid arthritis, added
almost another US $3 billion to its 2016 sales and posted nearly US $19 billion in revenues.
Last year, AbbVie’s raised expectations for Humira’s earnings to reach US $21 billion in global sales by 2020. The
company believes this drug will continue to be a significant cash contributor
until 2025 and the US $21 billion sales forecast
by 2020 is about US $3 billion higher than its expectation two years ago.
In 2016, the US Food and Drug Administration
(FDA) approved Amgen’s Amjevita (adalimumab-atto) — a biosimilar of Humira. And in 2017, another Humira biosimilar — Boehringer Ingelheim’s Cyltezo
(adalimumab-adbm) — received approval from the FDA and European authorities.
Click here to Access All the 2017 Data (Excel
version available) for FREE!
Enbrel (etanercept),
the longest-used biologic medicine for the treatment of rheumatism around the
world, was the second best-selling drug with US $8.262 billion in 2017 sales.
The sales of the drug were down from US $9.366 billion in
2016 owing to lower selling prices and increased
competition, which in turn hurt demand.
Since it was first approved in the United States in 1998,
Enbrel has been approved in over 100 countries and the drug is promoted by Amgen,
Pfizer
and Takeda
in different geographies.
Novartis’ biosimilar copy of Enbrel, which got approved by the FDA in August
2016 for the treatment of patients with
rheumatoid arthritis (RA), plaque psoriasis, ankylosing spondylitis (AS) and
other diseases is still not on the market because of a patent-protection
challenge from Amgen.
Amgen is arguing in the US federal court
that its drug has patent protection until 2029.
Click here to Access All the 2017 Data (Excel
version available) for FREE!
Fast-growing drugs: Eylea and Revlimid bring
fortunes for Regeneron and Celgene
Regeneron’s
flagship eye treatment, Eylea (aflibercept) which is marketed by Bayer outside the United States, added another US $1 billion in
annual sales last year to record US $8.260 billion in total sales. Eylea net
sales grew 11 percent year-on-year in the US and 19 percent year-over-year
outside the US.
The company believes much of the recent
growth in the US was driven by demographic trends with an aging population as
well as an overall increase in the prevalence of diabetes.
These demographic trends are expected to
continue in the coming years, providing an opportunity for continued growth.
Eylea sales alone contribute 63 percent to Regeneron’s total sales.
Click here to Access All the 2017 Data (Excel
version available) for FREE!
Celgene’s
Revlimid
(lenalidomide)
— a thalidomide derivative introduced in 2004 as an immunomodulatory agent for the treatment of various cancers such as multiple myeloma — brought in an additional US $1.2 billion in 2017 sales and had total revenues of US $8.187 billion.
Revlimid continues to contribute more than 60 percent to the company’s total sales of US $13 billion.
Celgene received a setback this month as the
USFDA refused to consider Celgene’s
application for ozanimod, an experimental
treatment for relapsing multiple sclerosis. The treatment was being seen as a
key to the company’s fortunes as Celgene had
said ozanimod is worth US $4 billion to
US $6
billion a year in peak sales.
Click here to Access All the 2017 Data (Excel
version available) for FREE!
Gilead’s Hepatitis C franchise enters free fall
Gilead Sciences’ blockbuster hepatitis C drugs franchise that includes Sovaldi and Harvoni continue to feel the
competitive heat as they registered US $9.137
billion in 2017 sales, down from US $14.834
billion the previous year.
While reporting 2017 results, Gilead provided guidance for
2018 and said its sales of Hepatitis C drugs could fall
further to US $3.5 billion - US $4 billion. At their peak in 2015, Gilead’s Sovaldi and Harvoni had together generated
US $19.1 billion in sales.
One of the major reasons for this drop is AbbVie’s launch of its new treatment Mavyret
at a deep price discount to the competition. AbbVie
also claims to have the shortest treatment course at eight weeks, compared with
12 weeks or longer for other treatments.
AbbVie reported US $1.274 billion in Hepatitis C drug sales
in 2017, down from US $1.522 billion in 2016.
Click here to Access All the 2017 Data (Excel
version available) for FREE!
Novartis’ Gleevec, Merck’s cardiovascular drugs, GSK’s Advair face generic heat
Novartis’ Gleevec (imatinib), which had at one point become the best-selling drug for Novartis and had brought in US $3.323 billion for the company in 2016, started facing generic competition last year and the anti-cancer drug lost US $1.380 billion in sales to bring in ‘only’ US $1.943 billion last year.
The US patents of Merck’s cardiovascular drugs — Zetia (Ezetimibe)
and Vytorin (Ezetimibe
and Simvastatin) — expired in April 2017. In May 2010, Merck and Glenmark
Pharmaceuticals entered into an agreement that allowed Glenmark to launch
a generic version of Zetia in late 2016. The drugs
that had combined sales of US $3.701
billion in 2016 felt the generic heat in 2017 and the sales were US
$1.606 billion lower at US $2.095
billion.
Click here to Access All the 2017 Data (Excel
version available) for FREE!
GSK’s Advair, which was expected
to encounter generic competition in 2017, continued to breathe easy as the FDA
found deficiencies in the applications of Hikma, Mylan and Sandoz.
All three failed to get the FDA nod for their generic versions of Advair, a drug used in the management of asthma and chronic obstructive pulmonary disease that generated sales worth US $4.431 billion (£3.130 billion) in 2017.
Top 15 drugs by sales
Here is PharmaCompass’ compilation
of the best-selling drugs of 2017. This is based on information extracted from
annual reports and US Securities and Exchange Commission (SEC) filings of major
pharmaceutical companies.
If you would like your own copy of all the information we’ve collected, email us at support@pharmacompass.com and we’ll send you an Excel version.
Click here to access all the 2017 data (Excel
version available) for FREE!
S. No.
Company / Companies
Product Name
Active Ingredient
Main Therapeutic Indication
2017 Revenue in Millions (USD)
1
AbbVie Inc., Eisai
Humira®
Adalimumab
Immunology (Organ Transplant, Arthritis etc.)
18,946
2
Amgen, Pfizer Inc., Takeda
Enbrel®
Etanercept
Immunology (Organ Transplant, Arthritis etc.)
8,262
3
Regeneron, Bayer
Eylea
Aflibercept
Ophthalmology
8,260
4
Celgene
Revlimid
Lenalidomide
Oncology
8,187
5
Roche
MabThera®/Rituxan®
Rituximab
Oncology
7,831
6
Johnson & Johnson, Merck, Mitsubishi Tanabe
Remicade®
Infliximab
Autoimmune Disorders
7,784
7
Roche
Herceptin®
Trastuzumab
Oncology
7,435
8
Bristol-Myers Squibb, Pfizer Inc.
Eliquis®
Apixaban
Cardiovascular Diseases
7,395
9
Roche
Avastin®
Bevacizumab
Oncology
7,089
10
Bayer, Johnson & Johnson
XareltoTM
Rivaroxaban
Cardiovascular Diseases
6,590
11
Bristol Myers Squibb, Ono Pharmaceutical
Opdivo
Nivolumab
Oncology
5,815
12
Sanofi
Lantus
Insulin Glargine
Diabetes
5,731
13
Pfizer Inc.
Prevnar 13/Prevenar 13
Pneumococcal 7-Valent Conjugate
Anti-bacterial
5,601
14
Pfizer Inc., Eisai
Lyrica
Pregabalin
Neurological/Mental Disorders
5,318
15
Amgen, Kyowa Hakko Kirin
Neulasta®
Pegfilgrastim
Blood Disorders
4,553
Sign up, stay ahead
In order to stay informed, and receive
industry updates along with our data compilations, do sign up for the PharmaCompass Newsletter and
you will receive updated information as it becomes available along with a lot
more industry analysis.
Click here to Access All
the 2017 Data (Excel version available) for FREE!
Impressions: 58406
In less than three weeks, Donald Trump will assume office as the
President of the United States. He has mentioned that he wants Medicare (a
national social insurance program) to directly negotiate the price it pays for prescription drugs.
Medicare provides health insurance to Americans aged 65 or more, who
have worked and paid into the system through the payroll tax. It also provides
health insurance to younger people with some disabilities or end-stage renal
disease and amyotrophic lateral sclerosis.
In 2015, Medicare provided health insurance to over 55 million Americans — including 46 million people aged 65 or more, and nine million younger people.
As we flag off the New Year, PharmaCompass
provides insights into drug prices and prescription patterns in the US in order
to help professionals make informed decisions. We believe that the cost of
medicines in the US, which have been a subject of much public outcry and
discussions in the recent years, will continue to be scrutinized during 2017.
Medicare data for 2014
Medicare Part D, also known as the Medicare prescription drug benefit — the program which subsidizes the costs of prescription drugs and prescription drug insurance premiums for Medicare beneficiaries — published a data set (for calendar year 2014) which contains information from over one million healthcare providers
who collectively prescribed approximately US $121 billion worth of prescription
drugs paid for under this program.
For each prescriber and drug, the dataset
includes the total number of prescriptions that were dispensed (including
original prescriptions and any refills), and the total drug cost.
The total drug cost includes the ingredient cost of the medication, dispensing fees, sales tax, and any applicable administration fees. It’s based on the amounts paid by the Part D plan, the Medicare beneficiary, other government subsidies, and any other third-party payers (such as employers and liability insurers).
The total drug cost does not reflect any manufacturer rebates paid to Part D plan sponsors through direct and indirect remuneration or point-of sale rebates. In order to protect the beneficiary’s privacy, the Centers for Medicare & Medicaid Services (CMS) did not
include information in cases where 10 or fewer prescriptions were dispensed.
Top
Ten Drugs by Cost, 2014 [Most expensive for Medicare]
Drug Name
Total Claim Count
Beneficiary Count
Prescriber Count
Total Drug Cost
Sofosbuvir
109,543
33,028
7,323
$3,106,589,192
Esomeprazole Magnesium
7,537,736
1,405,570
286,927
$2,660,052,054
Rosuvastatin Calcium
9,072,799
1,752,423
266,499
$2,543,475,142
Aripiprazole
2,963,457
405,048
130,933
$2,526,731,476
Fluticasone/Salmeterol
6,093,354
1,420,515
281,775
$2,276,060,161
Tiotropium Bromide
5,852,258
1,211,919
253,277
$2,158,219,163
Lantus
Solostar
(Insulin Glargine)
4,441,782
972,882
224,710
$2,016,728,436
Sitagliptin Phosphate
4,495,964
789,828
190,741
$1,775,094,282
Lantus
(Insulin Glargine)
4,284,173
787,077
223,502
$1,725,391,907
Lenalidomide
178,373
27,142
9,337
$1,671,610,362
View the Medicare Part D National Prescriber Summary Report, Calendar Year 2014 (Excel version available) for FREE!
Top
Ten Drugs by Average Cost per Claim, 2014 [Most expensive drugs]
Drug Name
Total Claim Count
Beneficiary Count
Prescriber Count
Total Drug Cost
Average Cost Per Claim
Adagen
13
$1,224,835
$94,218
Elaprase
100
$6,560,225
$65,602
Cinryze
1,820
194
196
$96,155,785
$52,833
Carbaglu
60
$2,901,115
$48,352
Naglazyme
129
$6,189,045
$47,977
Berinert
538
73
68
$25,685,311
$47,742
Firazyr
1,568
269
232
$70,948,143
$45,248
H.P. Acthar
9,611
2,932
1,621
$391,189,653
$40,702
Procysbi
314
41
47
$12,542,911
$39,946
Folotyn
15
$598,210
$39,881
Top
Ten Drugs by Claims, 2014 [Most Commonly Used by Patients]
Generic Name
Total Claim Count
Beneficiary Count
Prescriber Count
Total Drug Cost
Lisinopril
38,278,860
7,454,940
464,747
$281,614,340
Levothyroxine Sodium
37,711,869
6,245,507
416,518
$631,855,415
Amlodipine Besylate
36,344,166
6,750,062
451,350
$303,779,661
Simvastatin
34,092,548
6,768,159
387,651
$346,677,118
Hydrocodone-Acetaminophen
33,446,696
8,005,790
677,865
$676,296,988
Omeprazole
33,032,770
6,707,964
475,122
$529,050,385
Atorvastatin Calcium
32,603,055
6,740,061
419,327
$747,635,818
Furosemide
27,133,430
5,176,582
456,047
$135,710,772
Metformin HCl
23,475,787
4,509,978
364,273
$203,948,989
Gabapentin
22,143,641
4,298,609
486,754
$492,557,255
View the Medicare Part D National Prescriber Summary Report, Calendar Year 2014 (Excel version available) for FREE!
Top
Ten Drugs by Prescribers, 2014 [Most Popular with Doctors]
Generic Name
Total Claim Count
Beneficiary Count
Prescriber Count
Total Drug Cost
Hydrocodone/Acetaminophen
33,446,696
8,005,790
677,865
$676,296,988
Ciprofloxacin HCl
7,253,018
4,926,835
568,201
$46,728,353
Amoxicillin
6,298,980
4,384,899
557,614
$31,193,739
Cephalexin
5,040,219
3,529,303
557,048
$36,987,401
Azithromycin
7,339,954
5,274,010
544,625
$70,699,119
Prednisone
11,032,986
4,505,821
536,108
$86,537,932
Tramadol HCl
14,250,227
4,272,724
515,816
$125,343,514
Sulfamethoxazole /Trimethoprim
4,833,758
3,090,944
500,790
$29,231,511
Gabapentin
22,143,641
4,298,609
486,754
$492,557,255
Amoxicillin/Potassium Clav
3,551,452
2,710,244
478,361
$61,713,432
The findings from CMS
data
The CY 2014 data represented a 17 percent
increase compared to the 2013 data set and a substantial part of the total estimated prescription drug spending (as estimated by the Department of Health and Human Services Office of the Assistant Secretary for Planning and Evaluation, or ASPE) in the United States — at about US $ 457 billion in 2015, which was 16.7 percent of the overall personal healthcare services.
Of that US $ 457 billion, US $ 328 billion (71.9 percent) was for retail
drugs and US $ 128 billion (28.1 percent) was for non-retail drugs.
The drug pricing process in the US is complex and
reflects the influence of numerous factors, including manufacturer list prices,
confidential negotiated discounts and rebates, insurance plan benefit designs,
and patient choices.
An IMS study found that across 12 therapy classes widely used in Medicare Part D,
medicine costs to plans and patients in Medicare Part D are 35 percent below
list prices.
View the Medicare Part D National Prescriber Summary Report, Calendar Year 2014 (Excel version available) for FREE!
While the CMS does not
currently have an established formulary, Part D drug coverage excludes drugs
not approved by the US Food and Drug Administration, those prescribed for off-label
use, drugs not available by prescription for
purchase in the US, and drugs for which payments would be available under Parts
A or B of Medicare.
Part D coverage
excludes drugs or classes of drugs excluded from Medicaid coverage,
such as:
Drugs used for anorexia, weight loss, or weight gain
Drugs used to promote fertility
Drugs used for erectile dysfunction
Drugs used for cosmetic purposes (hair growth, etc.)
Drugs used for the symptomatic relief of cough and colds
Prescription vitamins and mineral products, except prenatal vitamins and fluoride preparations
Drugs where the manufacturer requires (as a condition of sale) any associated tests or monitoring services to be purchased exclusively from that manufacturer or its designee
Our view
The Medicare program is designed such that the
federal government is not permitted to negotiate prices of drugs with the drug
companies, as federal agencies do under other programs.
For instance, the Department of Veterans Affairs — which is allowed to negotiate drug prices and establish a formulary — has been estimated to pay (on an average) between 40 to 58 percent less for drugs, as opposed to Medicare Part D.
If Trump administration kick starts direct
negotiations on Medicare drug prices with drug companies, 2017 will surely turn
out to be a year for the pharmaceutical industry to remember.
View the Medicare Part D National Prescriber Summary Report, Calendar Year 2014 (Excel version available) for FREE!
Impressions: 7923
This week, Phispers brings you the
latest on the Ranbaxy-Daiichi and Merck-Gilead cases. There is also news on
Medtronics, which faces a whistleblower lawsuit. And Valeant, which has come
under the scanner for allegedly defrauding insurers. Our compliance roundup
updates you on companies across the world that faced regulatory action
recently. Singapore court’s 373-page order reveals how Ranbaxy withheld information from DaiichiLast week, a report in The Indian Express brought to light how Ranbaxy deliberately withheld information from Japan’s Daiichi Sankyo in the Ranbaxy-Daiichi case. The information was based on a copy of the Singapore International Arbitration Centre’s (SIAC) order, passed in April 2016. The former owners of Ranbaxy – Malvinder Singh and Shivinder Singh – face a penalty of Rs 35 billion (US $ 523 million) and have until August 22 to challenge the SIAC order. The information implicates the Ranbaxy top brass in a “in a slew of irregularities, from fraud to falsehood.” In over 373 pages, the SIAC order lays out what it calls “the path of deception that Ranbaxy took and how it kept Japan’s Daiichi Sankyo — which bought Ranbaxy in 2008 for Rs 198 billion (US $ 2.96 billion) — in the dark even a year after its purchase”. The SAIC order was based on a 2004 Self-Assessment Report (SAR) prepared by the then head of research and development of Ranbaxy, Rajinder Kumar, for the company’s internal use. The contents of an internal report were not shared with Daiichi. The SAR listed
over 200 drugs, including antiretroviral drugs for treating AIDS patients, for
which Ranbaxy allegedly used fabricated data to bag approvals from regulators
and authorities of more than 40 countries. Compliance roundup: Chinese, Indian, American and Spanish firms in compliance troubles Notice of non-compliance to Artemis Biotech: Artemis
Biotech, a division of Themis
Medicare in India, received a notice
of non-compliance from European regulators. According to the regulators,
the company had violated basic principles of data integrity within its instrument
laboratory. And the relevant GMP data was outside the control of the quality
management system. As an outcome of the inspection, the Certificates of Suitability (CEPs) granted for popular cholesterol lowering ingredient – simvastatin – have been suspended. Just three months ago another Indian manufacturer – Krebs
Biochemicals & Industries – had its CEPs suspended for the same product.Alcor found to have unsuitable facilities: A Spanish manufacturer – Alcor SL – that manufactures liquid syrups for use in Spain was found not to have suitable facilities, personnel and materials to ensure proper compliance with GMP during an inspection in June this year. Although the company responded with a corrective action plan, it was found “insufficient”.Claris recalls injections in the UK: Indian manufacturer Claris
Lifesciences recalled
Furosemide
injections in the United Kingdom as they had been inadvertently distributed in
the country. The product was intended for sale in Australia. FDA’s warning letters to Zhejiang Medicine, Concept Products: While
there was activity in Europe, the FDA issued a warning
letter to Zhejiang
Medicine (Xinchang Pharmaceutical Factory), a manufacturer of antibiotics
like levofloxacin,
daptomycin
and vancomycin,
for data integrity violations. Laboratory personnel were found “disguising testing”. The personnel were conducting unofficial testing that was being recorded in separate ‘R&D’ folders before conducting the officially reported sample analyses. Analysts were also found signing
and dating microbiological testing laboratory worksheets five days before the
test results were available and backdating laboratory worksheets for impurities
and content testing by four days.The FDA also issued a warning
letter to a Chinese manufacturer, Concept Products Limited, for “significant violations of cGMP regulations for finished pharmaceuticals”. It placed yet another Indian company Laxachem Organics and Chinese firm Yangzhou
Hengyuan on import alert. Warning letter to Noven: A US-based patch manufacturer – Noven
Pharmaceuticals – received a warning
letter over quality concerns uncovered in its transdermal drug delivery
systems (TDDS) such as Minivelle
and Daytrana.
The FDA expressed concerns over the scientific soundness of the company’s measurement method since the FDA stated that “your unsound methods could be masking product failures” and leading “to product detachment, expose the drug to other people, and other safety issues.” Now, Merck has to pay Gilead’s US $ 200 million legal feeIn March this year, Merck
had won a legal dispute over sofosbuvir,
the API in Gilead's
multibillion-dollar drugs Sovaldi and Harvoni.
The federal jury had ordered Gilead to pay Merck US $ 200 million in damages
for infringing on patents for the hepatitis C drugs. But
in June, the US Dristrict Judge Beth Labson Freeman threw out Merck’s victory and snatched back the US $ 200 million Merck had been awarded. Last week, the same judge added insult to Merck’s US $ 200 million-injury. Freeman said Gilead was entitled to relief from legal fees it had incurred while defending its case.Merck has been handed a US $200
million bill for Gilead's
legal fees. Merck now intends to appeal in the case, saying the judge’s ruling “does not reflect the facts of the case.” FDA launches improved web-based version of its Orange BookThis week, the US Food and Drug
Administration (FDA) launched an improved
web-based version of its Orange Book – a publication on drugs approved on the basis of safety and effectiveness. The Orange Book is widely used by doctors and by the regulatory community for identifying which drug products are substitutable for one another. The improved Orange Book has an updated design and has more user-friendly search optionsFormerly known as the Approved
Drug Products with Therapeutic Equivalence Evaluations, the Orange Book had
first appeared as a published list in 1980. It came online in 1997. Valeant allegedly defrauded insurers, may be under criminal
investigation In one of the most serious probes
faced by Valeant
Pharmaceuticals, the Canada-headquartered company may be under criminal investigation over allegations that it defrauded insurers by hiding its ties with a mail-order pharmacy – Philidor – that boosted its sales. Prosecutors are probing whether
Philidor made false statements to insurers about its ties with Valeant, while
helping patients get coverage for the higher-priced Valeant drugs. According to
a report published in The Wall Street
Journal, criminal charges are likely to be levied against former Philidor
executives and against Valeant as a company. The relationship between Philidor
and Valeant has been under the scanner since October 2015, when questions were
raised about Valeant's accounting. Novartis to expand capacity of monoclonal antibody plant in EuropeNovartis
is investing
US $ 100 million to expand its monoclonal antibody (mAb) capacity at a
plant in Europe. The Swiss drugmaker has committed about US $ 1 billion to
boost its biosimilar production in order to emerge a leading player in
biosimilars. Novartis is beginning work on the mAb project that will boost
capacity by 70 percent at the Novartis biotechnology center in Huningue,
France. Meanwhile, the company has
acknowledged that employees in South Korea may have been involved in rebate
trickeries. But it says an investigation of similar accusations
in Turkey uncovered no problems. In Turkey, Novartis considers the matter
closed.In April, a prosecutor in Turkey
had reportedly opened an investigation after receiving a copy of an email sent
by an anonymous whistleblower to Novartis CEO saying the unit there paid
consultants US $ 290,000 in 2013 and 2014 to win about US $ 85 million in
business from government hospitals.Matters in South Korea are a lot serious. In South Korea, prosecutors want the government to suspend the company’s operations there after they indicted half-dozen executives for issuing improper rebates. German watchdog criticizes efforts to accelerate new drug approvalsGermany’s cost-effectiveness watchdog – the German Institute for Quality and Efficiency in Health Care – has criticized
an effort by European regulators to accelerate approval for new medicines
based on limited evidence. These concerns come at a time when regulators on both
sides of the Atlantic are looking for new approaches to fulfill unmet
medical needs through faster approval of drugs.Adaptive pathways approach is a term
used to describe a method for jumpstarting drug approvals for select patient
populations. Two years ago, the European Medicines Agency (EMA) had launched a
specific pilot program in this direction. However, the German watchdog
maintained that the EMA failed to make its case that this approach for
approving drugs can make a demonstrable difference. Medtronic faces
whistleblower lawsuit for using devices under false pretensesMedtronic, a major medical device manufacturer, is facing a whistleblower lawsuit that claims it sought FDA approval for its devices under false pretenses. The devices were being regularly used for a purpose they weren’t intended to be used by the regulators.According to Dr. Vikas Saini, president of the Lown Institute, a Boston healthcare think tank, who has been following the case, the devices had been labelled ‘not for cervical spine use’. “Yet, in everything about them, including emails from their marketing folks, it makes clear that they were meant to be and were used in the cervical spine,” Saini said.Medical devices are lightly regulated by the FDA. Once
cleared by the FDA, physicians used medical devices however they deem fit. Questions being
raised on health of Clinton, Trump Donald Trump and Hillary Clinton are two of the oldest presidential candidates in the US history. While Clinton’s doctor certified that she “is in excellent physical condition” and Trump’s physician declared he would be “the healthiest president – ever”, these testaments are not being taken seriously in the absence of detailed medical records. Both Trump’s and Clinton’s doctors released brief
assessments of their health recently. Television host Sean Hannity has aired a series of segments on Fox that cast doubts on Clinton’s health. Democrats, on the other hand, have been questioning Trump’s mental health. One congresswoman recently suggested he should undergo a “mental fitness test.”
Impressions: 5118
GSK, Google form first bioelectronics firm; 11 generic companies benefit from the Teva Allergan deal
This week,
Phispers brings to you the details of the bioelectronics firm formed by GSK and
Google. There is also news on companies like Teva, Takeda, Jinan Jinda and Eli
Lilly, besides two other news snippets pertaining to the FDA -- while the first
one pertains to generic approvals, the other one relates to an additional black
box warning on a few antibiotics. GSK and Google
join hands to form first bioelectronics startupGlaxoSmithKline and Google’s parent company – Alphabet – have joined hands to create a new company that is focused on fighting diseases by targeting electrical signals in the human body. This way, GSK and Alphabet’s life sciences unit – known as Verily Life Sciences – will be jump-starting a new field of medicine known as bioelectronics.Verily Life
Sciences and GSK will together contribute US $ 715.12
million
over seven years to the startup Galvani Bioelectronics. The startup will develop
miniature electronic implants for the treatment of asthma, diabetes and other
chronic conditions. The
implantable devices developed by Galvani, which is owned 55 percent by GSK and
45 percent by Verily, can modify electrical nerve signals. The aim is to
modulate irregular or altered impulses that occur in many illnesses.The
new company
will be based at GSK’s Stevenage research center north of London, with a second research hub in South San Francisco.The announcement comes just weeks after GSK had said it was going to use Apple’s HealthKit to conduct clinical trials.Three years ago, GSK had first unveiled its ambitions in bioelectronics in the journal – Nature. Bioelectronic remedies attach battery-powered implants the size of a grain of rice (or even smaller) to individual nerves to correct faulty electrical signals between the nervous system and the body’s organs.GSK believes altering these nerve signals could open up the airways of asthma patients, reduce inflammation in the gut from Crohn’s disease and treat patients with a range of other chronic ailments such as arthritis. So far, the implants have only been tested on animals but the aim is to produce treatments that will supplement or replace drugs that often come with side-effects.GSK
has been working on bioelectronic medicines since 2012 in a push to develop new
patentable treatments, since its Advair respiratory treatment faces competition
from generic versions. It has invested US $50 million in a venture capital fund
for bioelectronics and provided funding to scientists working in the field. Teva divests 79
products to 11 generic players to close Allergan dealTeva
Pharmaceutical Industries – the world’s largest generics drug company – won a US
anti-trust approval to purchase Allergan's generics
business, after agreeing to divest 79 generic drugs to rival firms. This was arrived
at to settle Federal
Trade Commission (FTC) charges that its proposed US $ 40.5 billion acquisition of Allergan’s generic pharmaceutical business would be anti-competitive. The remedy requires Teva to divest the drug portfolio to 11 firms, and marks the largest drug divestiture order in a FTC pharmaceutical merger case.The Teva-Allergan deal, which was announced in July 2015, solidifies Teva’s position as the world's largest maker of generics while freeing Allergan to focus on branded drugs.The
companies that
have acquired
the divested products are Mayne Pharma
Group, Impax
Laboratories, Dr Reddy’s Laboratories, Sagent
Pharmaceuticals, Cipla Limited, Zydus Worldwide
DMCC, Mikah Pharma, Perrigo Pharma
International, Aurobindo
Pharma USA,
Prasco and 3M Company. Eli Lilly CEO
steps down; company under probe by US Justice Department Eli Lilly CEO John
Lechleiter has stepped down after steering the pharma company through long R&D droughts. The company’s president David Ricks will move up to the top spot. And after a brief spell as executive chairman, Lechleiter will leave the company next spring.Lechleiter
has been the company's CEO since April 1, 2008, and the chairman of its board
of directors since January 1, 2009.The
announcement has come at a time when Eli Lilly has been asked by the
Justice Department to disclose information on relationships with pharmacy benefits
managers (PBMs), the companies that negotiate prices and set reimbursement
conditions.It
has not been clear what exactly the department of justice is looking for. In
the past, drug makers such as Novartis and AstraZeneca have agreed to
pay fines and penalties to settle allegations pertaining to PBMs. FDA continues
to race ahead with generic approvals The
American regulator has reduced its pile of ANDA (abbreviated new drug
applications) by about 500
applications in the first six months of 2016. The FDA has also approved 315 more ANDAs over the same time period and has sent 66 more complete response letters — or rejections — to drug makers.This
news comes after Bloomberg reported
last month that the FDA has become ‘something of a bogeyman’ for India’s stock markets by approving generic drug applications from India at a record place. Similarly, PharmaCompass
had reported last week that Indian
companies have been fixing compliance issues. China’s Jinan Jinda fails another EDQM inspection; compliance troubles in Denmark In
regulatory news from across the world, Jinan Jinda, a Chinese API
manufacturer that had failed an inspection by Italian regulators in June 2015,
had more bad news awaiting it a year on. In
a June 2016
re-inspection, this time by the Spanish Health Authority, the regulator maintained the ‘facilities non-compliance standing’ since two critical observations were made and the corrections from the previous inspection “were found as not having been implemented in a satisfactory way”. And critical deficiencies were found on raw data.In
the June 2015 inspection, the critical observation was related to an unofficial
and non-controlled storage area containing mainly raw materials and finished
products which had been made
inaccessible to inspectors as the door had been removed and replaced with a panel fixed with
screws to the wall.Meanwhile,
the FDA issued an untitled letter (dated July 15, 2016) to Danish allergy
immunotherapy company ALK-Abelló (ALK) over manufacturing and quality control issues at its Horsholm, Denmark facility. The letter comes after a 12-day inspection of the facility in March 2016. During the inspection, the FDA had cited ALK for four “significant deviations” from cGMP requirements. Another black
box warning added to antibiotics like Cipro and LevaquinThe
FDA has upgraded
warnings on
certain antibiotics, such as Johnson & Johnson’s Levaquin, Bayer’s Cipro
extended-release tablets and Merck’s Avelox. The FDA had
added a black box warning in 2008 about the increased risk of tendinitis in
which the tissue connecting muscle to bone becomes inflamed. In
May this year, the FDA had advised restricting the use of fluoroquinolone antibiotic for certain uncomplicated
infections and had warned about the disabling side-effects of the drug.The new warning talks about long-term risks to the drugs’ current black box warning. The agency also advised using the drugs only for serious infections. Manufacturers of fluoroquinolone have faced thousands of lawsuits from patients who claim that their injuries were caused by the drugs. J&J alone faced 3,400 lawsuits over Levaquin’s links to tendon problems and has also settled many of those cases. Takeda to
overhaul R&D, downsize operations in the UKTakeda Pharmaceutical of Japan has
said it plans to build a new pipeline of drugs. It plans to revamp its
research operations at the cost of around US $ 727 million.. The
company also plans to close some of its R&D operations in the UK. Takeda is
beginning the first ‘consultation stage’ of the layoff process in the UK, which hosts a pre-clinical R&D operation in Cambridge as well as a development center headquarter with facilities in the UK, Switzerland and Denmark.Under the revamp, Takeda’s R&D activities will be concentrated in Japan and the US, the 235-year old drug company said in a statement. Takeda plans to now focus on the three therapeutic areas of oncology, gastroenterology and the central nervous system.“We need to first build new capabilities and embrace new ways of working,” Andy Plump, Takeda’s chief medical and scientific officer, said in the statement.
Impressions: 2757
The proposed overhaul of the U.S. drug
approval system through the 21st
Century Cures Act won’t take us back in time. With the right safeguards in
place, this legislation can help win the fight against Alzheimer’s, Ebola and many
other diseases. It seems like the much awaited prescription for patients as
well as the industry.Development of pharmaceutical drugs can be
time-consuming and extremely expensive. The last industry-funded research estimated
the cost of developing
each new drug at US $ 2.6 billion. The losing fight against Alzheimer’sOn top of the high cost of development, the failure rate can be quite high. Take the case of Alzheimer’s disease. Since 1998, over 120 programs attempting to develop Alzheimer’s drugs have failed. Between 2002 and 2012, there was just a 0.04 percent success rate of Alzheimer’s drugs meeting the standard.Last week, the fight against Alzheimer’s received yet another setback. US-based Biogen’s biotech drug, Aducanumab, was hailed as a potential breakthrough in March when it became the first Alzheimer's treatment to significantly slow cognitive decline. However, new
data published by Biogen last week revealed that Aducanumab failed to
significantly slow mental decline, thereby tempering the great expectations the
world had from this potential drug. Enter
the 21st Century Cures ActClearly, the new drug development process
needs to change. On July 10, the U.S. House of Representatives approved
the 21st Century Cures Act. The Act seeks to modernize clinical trials and
deliver better, faster cures to more patients. The Act is awaiting approval in
the Senate. However, several provisions of the proposed
act have come under severe criticism. For instance, in The New England Journal of
Medicine, Jerry Avorn, M.D., and Aaron S. Kesselheim, M.D., J.D., M.P.H., say “if enacted into law, some of its provisions could have a profound effect on what is known about the safety and efficacy of medical products, as well as which ones become available for use.” Biomarker
based drug approvalThe proposed Act encourages biomarker-based drug approval. Unlike conventional drug approvals, which involve extensive clinical trials, where outcomes are studied in patients, the biomarker-based approach works on studying a “surrogate marker”, which is strongly linked with the final outcome in the patient. “A commonly used example
is cholesterol. While elevated cholesterol levels increase the likelihood for heart disease, the relationship is not linear – many people with normal cholesterol develop heart disease, and many with high cholesterol do not. ‘Death from heart disease’ is the endpoint of interest, but ‘cholesterol’ is the surrogate marker. A clinical trial may show that a particular drug (for example, simvastatin) is effective in reducing cholesterol, without showing directly that the drug prevents death.” The bill would encourage the FDA to rely
more on biomarkers and other surrogate measures rather than actual clinical
endpoints in assessing the efficacy of both drugs and devices. The biomarker-based drug approval has also
generated its fair share of concerns since “a drug's effect on a biomarker can make approval quicker and less costly, especially if the comparator is placebo, it may not always predict the drug's capacity to improve patient outcomes” But the key point to note is that the FDA
already uses surrogate endpoints in about half of the new drug approvals. The
legislation would not immediately change FDA approval standards, but they would
give the agency greater discretion to approve drugs on the basis of less comprehensive
data. Accelerated
approvals have been in place since 1992The FDA has had an Accelerated
Approval (AA) program in place since 1992. This has already been used
extensively in oncology
and HIV drugs.And the AA programme has benefited
patients. As per an FDA presentation, almost 90 percent of the drugs approved
for oncology through the AA process demonstrated a benefit in the post-market
study. Since 1995, 49 new drug indications have been launched. In the case of HIV
drugs, all indications that were given accelerated approval also received regular
approval.For something as dangerous as Ebola, where there still isn’t an approved treatment to contain the spread of the Ebola virus, computational models have been used to evaluate existing drugs. Over 50 FDA approved
drugs have now been identified with activity against the Ebola virus. Will it not be wise to use them in the fight
against Ebola? Need
for vigorous safety monitoringThe essence of the proposed 21st Century Cures Act is positive. Increased and faster drug approvals is great news for patients and the industry. What is needed are the right measures and safeguards to ensure the drug approvals (based on biomarkers) are not ‘free for all’ and there isn’t an unfettered access of such drugs to a large population.More importantly, the proposed act will increase
safety monitoring. This means drug developers will have to monitor patients
more vigorously using devices, such as smartphones. If drugs get approved based
on biomarkers, the smartphone should be leveraged as an integrated health tool
that sends feedback on how patients are performing after a drug is
administered. Our
View There are several smartphone applications available today. For instance, Apple’s ResearchKit
allows developers to monitor multiple healthcare outcomes like asthma occurrence, determine the progress of Parkinson’s, share information about the effects of chemotherapy used in breast cancer and also measure gait and balance.Supportive legislation coupled with Apple
and Google aggressively getting into the healthcare space, it is just a matter
of time before the digital health revolution joins our fight against diseases
to benefit patients as well as the industry.
Impressions: 2430
It doesn’t help that the company, which calls
itself Merck in the United States isn’t allowed to do so in other parts of the
world, because the other Merck is called Merck. Confused? Well you
wouldn’t be the only one!
Merck is the world’s oldest pharmaceutical and chemical company, which has done business for almost 350 years. Since almost everybody in a chemistry lab has used a Merck product at some point of time, and now that Sigma Aldrich also belongs to Merck, we thought it would be worthwhile to create a simple comparison chart to better understand the two companies:
Merck & Co., Inc.
Merck KGaA
Website
www.merck.com
www.merckgroup.com
Logo
Headquarter
Kenilworth, New Jersey, United States
Darmstadt, Germany
Founded
1891, by George Merck, as an American
subsidiary of German Merck
1668, by Friedrich Jacob Merck
The Split
Owing to World War I, Merck &
Co. was expropriated by the U.S. government in 1917
Name in the US and Canada
Merck
EMD Millipore
(Emanuel Merck Darmstadt)
Name in the rest of the World
MSD,
Merck Sharp & Dohme or
MSD
Sharp & Dohme
Merck
Total Sales (2014)
$42 Billion
€11.5 Billion
Employees
70,000
39,000
Merger & Acquisitions
Sharp & Dohme, Inc.
Schering-Plough
Idenix Pharmaceuticals
Cubist Pharmaceuticals
OncoEthix
Medco Containment Services Inc.
Millipore Corporation
Serono SA
Sigma-Aldrich
AZ Electronic Materials SA
Major Products
Januvia®, Janumet®, Zetia®, Vytorin®, Gardasil®, Remicade®
Rebif®, Erbitux®, Gonal-f®, Concor®, Glucophage®, Euthyrox®
Recent facts of Confusion:
2011 German Merck KGaA used to have Facebook page:http://www.facebook.com/merck, but one day found U.S. Merck & Co. there instead. As Merck KGaA had an agreement with Facebook, the matter reached the courts
. Eventually Facebook admitted their mistake and let the German
Merck resume its place at www.facebook.com/merck.
U.S. Merck & Co. now sits at http://www.facebook.com/MerckBeWell.
2014 Protestors from the
group STOPAIDS (a network working
on how to secure an effective global response to HIV and AIDS) reached the offices of Merck KGaA in London, when protesting Merck’s “campaign to delay South Africa’s proposal to allow low-cost copies of patented drugs.”
The only problem: the protestors were targeting U.S. based Merck & Co instead of German Merck…
2014 Bloomberg headline “Bayer to Buy Merck Consumer-Health Unit for $14.2 Billion” resulted in Merck KGaA issuing a same day clarification “Merck to Keep Consumer Health Business” since the Bloomberg article was referring to the U.S. Merck & Co!
2015 Even Merck KGaA CEO Karl-Ludwig Kley admits that his company bears some fault in allowing the situation to get to this point: “over many decades we underinvested in our brand,” he told the Financial
Times: “we need to make people more aware of the fact there are two Mercks.”
RadioCompass just thought it was
worth helping Mr Kley out with this complilation!
Impressions: 9771