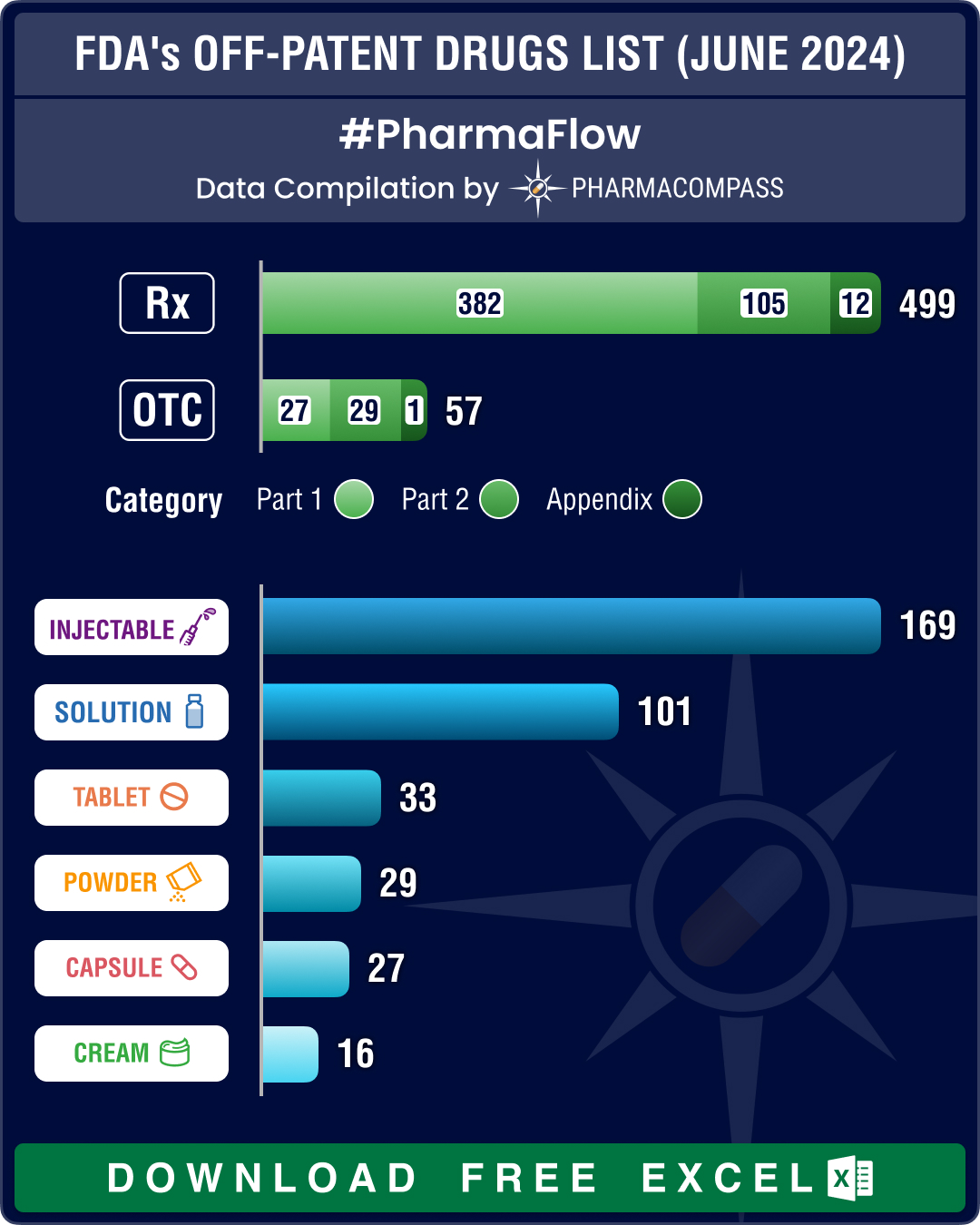
FDA’s December 2025 OPOE list features 784 prescription drugs, 73 OTC drugs
This
week, PharmaCompass brings you key highlights of the US Food and Drug Administration’s D
US drug shortages hit record high in Q1 2024, impacts cancer, ADHD drugs; Lilly, Novo ramp up production
Drug shortages are threatening healthcare systems the world over.
Be it the US, Canada, Europe or A


 Market Place
Market Place Sourcing Support
Sourcing Support