
Synopsis
0
VMF
0
Weekly News Recap #Phispers
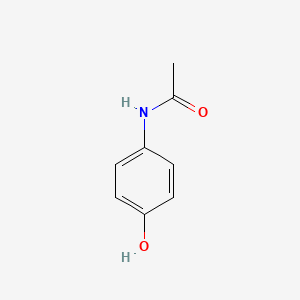

1. Acamol
2. Acephen
3. Acetaco
4. Acetamidophenol
5. Acetominophen
6. Algotropyl
7. Anacin 3
8. Anacin-3
9. Anacin3
10. Apap
11. Datril
12. Hydroxyacetanilide
13. N-(4-hydroxyphenyl)acetanilide
14. N-acetyl-p-aminophenol
15. P-acetamidophenol
16. P-hydroxyacetanilide
17. Panadol
18. Paracetamol
19. Tylenol
1. Paracetamol
2. 4-acetamidophenol
3. 103-90-2
4. Tylenol
5. N-(4-hydroxyphenyl)acetamide
6. Apap
7. Panadol
8. N-acetyl-p-aminophenol
9. 4'-hydroxyacetanilide
10. Acetaminofen
11. Datril
12. P-hydroxyacetanilide
13. P-acetamidophenol
14. Algotropyl
15. Lonarid
16. Naprinol
17. Doliprane
18. Injectapap
19. Acamol
20. Acenol
21. Anelix
22. Multin
23. P-acetaminophenol
24. Abensanil
25. Acetagesic
26. Acetalgin
27. Biocetamol
28. Clixodyne
29. Gelocatil
30. Liquagesic
31. Pyrinazine
32. Servigesic
33. Acephen
34. Alvedon
35. Anaflon
36. Apamide
37. Dymadon
38. Febridol
39. Febrilix
40. Febrolin
41. Finimal
42. Homoolan
43. Lestemp
44. Paracet
45. Tabalgin
46. Tralgon
47. Tussapap
48. Valadol
49. Valgesic
50. Vermidon
51. Alpiny
52. Amadil
53. Anhiba
54. Calpol
55. Dirox
56. Eneril
57. Fendon
58. Hedex
59. Lyteca
60. Neopap
61. Pacemo
62. Panets
63. Parmol
64. Tapar
65. Tempra
66. Acetamide, N-(4-hydroxyphenyl)-
67. Paracetamolo
68. Dafalgan
69. Dolprone
70. Momentum
71. Ortensan
72. Paldesic
73. Banesin
74. Captin
75. Disprol
76. Enelfa
77. Salzone
78. Exdol
79. P-acetylaminophenol
80. Febro-gesic
81. Nebs
82. Paracetamolum
83. Dolgesic
84. Elixodyne
85. Febrectal
86. Phenaphen
87. Tempanal
88. Abenol
89. Apacet
90. Apadon
91. Cetadol
92. Fensum
93. Janupap
94. Minoset
95. Napafen
96. Neodol
97. Nobedon
98. Pacemol
99. Panodil
100. Parapan
101. Pedric
102. Phendon
103. Rounox
104. Suppap
105. Korum
106. Pinex
107. Temlo
108. 4-(acetylamino)phenol
109. Ben-u-ron
110. Dial-a-gesic
111. Anacin-3
112. Calmanticold
113. Codoliprane
114. Demogripal
115. Dolegrippin
116. Doloreduct
117. Dristancito
118. Duracetamol
119. Eu-med
120. Grippostad
121. Gynospasmine
122. Medocodene
123. Paedialgon
124. Paracetanol
125. Parakapton
126. Pediapirin
127. Phenipirin
128. Phogoglandin
129. Predualito
130. Sanicopyrine
131. Scentalgyl
132. Sunetheton
133. Tachiprina
134. Termalgine
135. Treuphadol
136. Abrolet
137. Acertol
138. Acetamol
139. Acetofen
140. Afebrin
141. Afebryl
142. Aferadol
143. Algesidal
144. Algomol
145. Alpinyl
146. Analter
147. Antidol
148. Apitrelal
149. Atralidon
150. Babikan
151. Bacetamol
152. Cadafen
153. Calapol
154. Causalon
155. Cefalex
156. Codabrol
157. Codalgin
158. Codapane
159. Codicet
160. Codisal
161. Cofamol
162. Cosutone
163. Cuponol
164. Curadon
165. Custodial
166. Darocet
167. Daygrip
168. Deminofen
169. Democyl
170. Desfebre
171. Dimindol
172. Dolefin
173. Dolofugin
174. Dolorfug
175. Dolorstop
176. Dolotec
177. Dorocoff
178. Dularin
179. Durapan
180. Ecosetol
181. Excipain
182. Fanalgic
183. Farmadol
184. Febranine
185. Febrectol
186. Febricet
187. Febrinol
188. Fepanil
189. Finiweh
190. Fluparmol
191. Geluprane
192. Ildamol
193. Inalgex
194. Infadrops
195. Kataprin
196. Labamol
197. Lekadol
198. Lemgrip
199. Lupocet
200. Magnidol
201. Malidens
202. Maxadol
203. Mexalen
204. Minafen
205. Miralgin
206. Nealgyl
207. Neodolito
208. Neotrend
209. Neuridon
210. Nodolex
211. Ofirmev
212. Oralgan
213. Oxycocet
214. Pacimol
215. Panacete
216. Panadeine
217. Panadiene
218. Panaleve
219. Panamax
220. Panasorbe
221. Panofen
222. Pantalgin
223. Paracemol
224. Paracenol
225. Paracetol
226. Paracin
227. Paracod
228. Paracodol
229. Parador
230. Paradrops
231. Paralen
232. Paralief
233. Paralink
234. Paralyoc
235. Paramol
236. Paramolan
237. Paranox
238. Parasedol
239. Parasin
240. Paraspen
241. Parcetol
242. Parogal
243. Pediatrix
244. Perfalgan
245. Piramin
246. Pirinasol
247. Polmofen
248. Predimol
249. Prontina
250. Puernol
251. Pulmofen
252. Pyrigesic
253. Pyromed
254. Remedol
255. Rivalgyl
256. Rubophen
257. Rupemol
258. Sanicet
259. Schmerzex
260. Sedalito
261. Semolacin
262. Seskamol
263. Setakop
264. Setamol
265. Sifenol
266. Sinaspril
267. Sinedol
268. Stanback
269. Stopain
270. Supofen
271. Tazamol
272. Termacet
273. Termalgin
274. Termofren
275. Titralgan
276. Tricoton
277. Upsanol
278. Utragin
279. Veralgina
280. Viruflu
281. Vivimed
282. Zatinol
283. Abrol
284. Algina
285. Anapap
286. Andox
287. Asetam
288. Asomal
289. Aspac
290. Asplin
291. Benmyo
292. Curpol
293. Dhamol
294. Dolcor
295. Dolko
296. Dresan
297. Dypap
298. Febrex
299. Febrin
300. Lemsip
301. Malgis
302. Oltyl
303. Paceco
304. Pacet
305. Paedol
306. Painex
307. Pamol
308. Panex
309. Parake
310. Paroma
311. Plicet
312. Prodol
313. Reliv
314. Scanol
315. Setol
316. Sinmol
317. Tiffy
318. Tylex
319. Tylol
320. Tymol
321. Verpol
322. Volpan
323. Zolben
324. Neocitran
325. Nilnocen
326. Nina
327. Rubiemol
328. Vips
329. Supadol Mono
330. Treupel Mon
331. Bickie-mol
332. Fortalidon P
333. Gattaphen T
334. Gripin Bebe
335. Influbene N
336. Lonarid Mono
337. Lyteca Syrup
338. Panadeine Co
339. Dymadon Co
340. Toximer P
341. Treupel N
342. Accu-tap
343. 4-acetaminophenol
344. Helon N
345. Malex N
346. Spalt N
347. Tylex Cd
348. N-acetyl-4-aminophenol
349. Sk-apap
350. Paracetamole
351. Conacetol
352. Darvocet
353. Empracet
354. Panasorb
355. A-per
356. Apamid
357. Parelan
358. Prompt
359. Vicodin
360. Fevor
361. Freka-cetamol
362. Codisal Forte
363. Croix Blanche
364. Dolorol Forte
365. Dymadon Forte
366. Junior Disprol
367. Kinder Finimal
368. Liquigesic Co
369. Mono Praecimed
370. Percocet-demi
371. Perdolan Mono
372. Rockamol Plus
373. Viclor Richet
374. Actifed Plus
375. Kratofin Simplex
376. Neo-fepramol
377. Paracetamol Al
378. Paracetamol Bc
379. Paracetamol Pb
380. Acetanilide, 4'-hydroxy-
381. Claradol Codeine
382. Geralgine-p
383. Melabon Infantil
384. Migraleve Yellow
385. Paracetamol Saar
386. Pyregesic-c
387. Anti-algos
388. Para-suppo
389. Pasolind N
390. Supramol-m
391. No-febril
392. Panado-co
393. Para-tabs
394. Paracetamol Hexal
395. Paracetamol Raffo
396. Paracetamol Rosch
397. Paracetamol Stada
398. Dol-stop
399. Anadin Dla Dzieci
400. P-hydroxyphenolacetamide
401. Percocet-5
402. Cod-acamol Forte
403. Contra-schmerz P
404. Hy-phen
405. Medinol Paediatric
406. Paracetamol Basics
407. Panado-co Caplets
408. Paracetamol Von Ct
409. Pe-tam
410. Paracetamol Fecofar
411. Paracetamol Harkley
412. Paracetamol Heumann
413. Paracetamol Nycomed
414. Codral Pain Relief
415. Paracetamol Hanseler
416. Paracetamol Winthrop
417. 4-hydroxyacetanilide
418. Phenaphen W/codeine
419. Spalt Fur Die Nacht
420. A.f. Anacin
421. Capital With Codeine
422. Paracetamol Genericon
423. Anexsia
424. Demilets
425. Efferalgan
426. Endecon
427. Intensin
428. Naldegesic
429. Propacet
430. Resfenol
431. Theraflu
432. Wygesic
433. Paracetamol Ratiopharm
434. Coricidin Sinus
435. Paracetamol Italfarmaco
436. Sudafed Sinus
437. Coricidin D
438. Paracetamol Dc
439. Quiet World
440. Paracetamol Antipanin P
441. St Joseph Aspirin-free
442. New Cortal For Children
443. Infants' Feverall
444. St. Joseph Fever Reducer
445. Midol Teen Formula
446. Paracetamol Dr. Schmidgall
447. Acetamide, N-(p-hydroxyphenyl)-
448. P-hydroxy-acetanilid
449. Aspirin-free Anacin
450. Children's Tylenol Chewable
451. Nci-c55801
452. Pcm Paracetamol Lichtenstein
453. Tylenol Allergy Sinus
454. P-(acetylamino)phenol
455. Rhinex D-lay Tablets
456. Acetaminophene
457. Midol Regular Strength
458. Paracetamol Smithkline Beecham
459. Scherzatabletten Rezeptur 534
460. Percogesic With Codeine
461. 4-hydroxyanilid Kyseliny Octove
462. Bayer Select Head Cold
463. Bayer Select Allergy-sinus
464. Bayer Select Headache Pain
465. Dristan Cold No Drowsiness
466. Prestwick_13
467. St Joseph Aspirin-free For Children
468. 4-acetylaminophenol
469. Children's Acetaminophen Elixir Drops
470. Mfcd00002328
471. Children's Acetaminophen Oral Solution
472. Midol Pm Night Time Formula
473. Triaminic Sore Throat Formula
474. N-(4-hydroxyphenyl)ethanamide
475. Bayer Select Sinus Pain Relief
476. Phenol, P-acetamido-
477. Sine-off Sinus Medicine Caplets
478. Chebi:46195
479. Roxicet 5/500
480. Tocris-1706
481. Nsc-3991
482. N-acetyl-para-aminophenol
483. 4-(n-acetylamino)phenol
484. Acetaminophen (4-hydroxyacetanilide)
485. Bayer Select Menstrual Multi-symptom
486. Acetaco
487. N-acetyl-4-hydroxyaniline
488. St. Joseph Cold Tablets For Children
489. Chembl112
490. Nsc-109028
491. N-(4-hydroxyphenyl)acetamide (tylenol)
492. N-(4-hydroxyphenyl)-acetamide
493. 362o9itl9d
494. Aminofen
495. Dtxsid2020006
496. Atasol
497. Duaneo
498. Duorol
499. Component Of Dialog
500. Component Of Dilone
501. Fever All
502. Paracetamol (inn)
503. Component Of Endecon
504. Component Of Percocet
505. Component Of Phenaphen
506. Tyl
507. Component Of Percogesic
508. Dsstox_cid_6
509. Ncgc00016361-07
510. Acetominophen
511. Actamin
512. Cas-103-90-2
513. Pasolind
514. Redutemp
515. Robigesic
516. Valorin
517. Aceta Elixir
518. Paracetamol [inn]
519. Dafalgan Codeine
520. Jin Gang
521. Wln: Qr Dmv1
522. Dsstox_rid_75318
523. Dsstox_gsid_20006
524. Component Of Hycomine Compound
525. Acetavance
526. Paracetamolo [italian]
527. Calonal
528. Flexsure
529. Acenol (pharmaceutical)
530. N-(4-hydroxyphenyl)acetanilide
531. Drixoral Sinus
532. Aceta Tablets
533. Paracetamol [inn:ban]
534. Valorin Extra
535. Ccris 3
536. Snaplets-fr
537. Oraphen-pd
538. Phenaphen Caplets
539. Paracetamolum [inn-latin]
540. Tylenol (caplet)
541. Tylenol (geltab)
542. Tylenol 8-hour
543. Smr000112065
544. Tavist Allergy/sinus/headache
545. Dapa X-s
546. Drixoral Cold & Flu
547. Hsdb 3001
548. Sr-01000597517
549. Einecs 203-157-5
550. 222 Af
551. Acetaminophen [usp:jan]
552. Paracetamol (acetaminophen)
553. Nsc 109028
554. Acetominophene
555. Unii-362o9itl9d
556. 4-hydroxyanilid Kyseliny Octove [czech]
557. Children's Acetaminophen Elixir Solution
558. Claratal
559. Daphalgan
560. Resprin
561. Apacet Capsules
562. Atasol Caplets
563. Atasol Tablets
564. Tempra Caplets
565. Tylenol Caplets
566. Tylenol Elixir
567. Tylenol Gelcaps
568. Tylenol Tablets
569. Actamin Extra
570. Actamin Super
571. Aminofen Max
572. Anexsia 10/660
573. Apacet Elixir
574. Atasol Drops
575. Exdol Strong
576. P-acetoaminophen
577. Tempra Drops
578. Tempra Syrup
579. Tylenol Drops
580. Alpha-per
581. Citramon P
582. Excedrin Caplets
583. Dial-alpha-gesic
584. Apo-acetaminophen
585. 4-acetominophenol
586. Genebs X-tra
587. Paracetamol;tylenol
588. 4-acetamido Phenol
589. 4-acetamido-phenol
590. Tempra D.s
591. Apap, Paracetamol
592. P-hydroxyacetoanilide
593. Tylenol (tn)
594. Supac (salt/mix)
595. Tylox (salt/mix)
596. Zydone (salt/mix)
597. Atasol Forte Caplets
598. Atasol Forte Tablets
599. Atasol Oral Solution
600. Para-acetylaminophenol
601. Anexsia (salt/mix)
602. Endecon (salt/mix)
603. Sinubid (salt/mix)
604. Talacen (salt/mix)
605. Vicodin (salt/mix)
606. Wygesic (salt/mix)
607. Acetaminophen Uniserts
608. Datril Extra-strength
609. Tylenol Infants Drops
610. Demilets (salt/mix)
611. Empracet (salt/mix)
612. Intensin (salt/mix)
613. Propacet (salt/mix)
614. Suppap-120
615. Suppap-325
616. Suppap-650
617. Panadol Extra Strength
618. Theraflu (salt/mix)
619. Coricidin (salt/mix)
620. Liquiprin (salt/mix)
621. Hy-phen (salt/mix)
622. Iv-apap
623. Phenol Derivative, 11
624. Spectrum_000016
625. Tempra Chewable Tablets
626. Naldegesic (salt/mix)
627. Actimol Chewable Tablets
628. Feverall Junior Strength
629. Darvocet-n (salt/mix)
630. Anacin-3 Extra Strength
631. Liquiprin Infants" Drops
632. N-acetyl Para Aminophenol
633. Prestwick0_000868
634. Prestwick1_000868
635. Prestwick2_000868
636. Prestwick3_000868
637. Spectrum2_000085
638. Spectrum3_000283
639. Spectrum4_000140
640. Spectrum5_000736
641. Coricidin D (salt/mix)
642. Quiet World (salt/mix)
643. Genapap Children's Elixir
644. Tylenol Children's Elixir
645. 4-acetamidophenol, 98%
646. Actifed Plus (salt/mix)
647. Acetaminophen [mi]
648. Paracetamol [iarc]
649. Epitope Id:117710
650. Genapap Children's Tablets
651. Sudafed Sinus (salt/mix)
652. Acetaminophen [jan]
653. Ec 203-157-5
654. Actimol Infants' Suspension
655. Drixoral Sinus (salt/mix)
656. Liquiprin Children's Elixir
657. Schembl3480
658. Acetaminophen (jp17/usp)
659. Acetaminophen [hsdb]
660. Acetaminophen [inci]
661. Coricidin Sinus (salt/mix)
662. N-(4-hydroxyfenyl)ethanamid
663. Paracetamol [mart.]
664. Acetaminophen [vandf]
665. Bspbio_000915
666. Bspbio_001786
667. Dds-06a
668. Kbiogr_000560
669. Kbioss_000356
670. Paracetamol [who-dd]
671. Paracetamol [who-ip]
672. 4-13-00-01091 (beilstein Handbook Reference)
673. Actimol Children's Suspension
674. Apacet Extra Strength Caplets
675. Apacet Extra Strength Tablets
676. Aspirin-free Excedrin Caplets
677. Genebs Extra Strength Caplets
678. Mls001146925
679. Mls001331684
680. Mls002154041
681. Bidd:gt0005
682. Divk1c_000660
683. Spectrum1500101
684. Genapap Extra Strength Caplets
685. Genapap Extra Strength Tablets
686. Spbio_000010
687. Spbio_002836
688. Tapanol Extra Strength Caplets
689. Tapanol Extra Strength Tablets
690. Tylenol Extra Strength Caplets
691. Tylenol Extra Strength Gelcaps
692. Tylenol Extra Strength Tablets
693. Acetaminophen [usp-rs]
694. Actimol Junior Strength Caplets
695. Apacet Regular Strength Tablets
696. Bpbio1_001007
697. Excedrin Extra Strength Caplets
698. Genebs Regular Strength Tablets
699. Gtpl5239
700. Panadol Junior Strength Caplets
701. Sgcut00014
702. Tylenol Junior Strength Caplets
703. Midol Teen Formula (salt/mix)
704. Genapap Regular Strength Tablets
705. Panadol Maximum Strength Caplets
706. Panadol Maximum Strength Tablets
707. Schembl19474893
708. Tylenol Regular Strength Caplets
709. Tylenol Regular Strength Tablets
710. Aspirin-free Anacin (salt/mix)
711. Bdbm26197
712. Hms502a22
713. Kbio1_000660
714. Kbio2_000356
715. Kbio2_002924
716. Kbio2_005492
717. Kbio3_001286
718. Nsc3991
719. Tylenol Arthritis Extended Relief
720. Acetaminophen, Analytical Standard
721. Ninds_000660
722. Tylenol Infants" Suspension Drops
723. Bcpp000441
724. Drixoral Cold & Flu (salt/mix)
725. Hms1570n17
726. Hms1920a03
727. Hms2091g03
728. Hms2097n17
729. Hms2269g20
730. Hms3268a10
731. Hms3412d16
732. Hms3676d16
733. Hms3714n17
734. Paracetamol [ep Monograph]
735. Pharmakon1600-01500101
736. Tylenol Allergy Sinus (salt/mix)
737. Acetaminophen [orange Book]
738. Midol Regular Strength (salt/mix)
739. Act06727
740. Allay Component Acetaminophen
741. Amy39958
742. Bcp23431
743. Bucet Component Acetaminophen
744. Esgic Component Acetaminophen
745. Norco Component Acetaminophen
746. Nsc 3991
747. Str00901
748. To_000023
749. Triad Component Acetaminophen
750. Tylenol Children's Chewable Tablets
751. Tylox Component Acetaminophen
752. Acetaminophen [usp Impurity]
753. Acetaminophen, Bioxtra, >=99.0%
754. Bayer Select Head Cold (salt/mix)
755. Robitussin Night Relief (salt/mix)
756. Tox21_110397
757. Tox21_201930
758. Tox21_300100
759. Ac8790
760. Acetaminophen [usp Monograph]
761. Bancap Component Acetaminophen
762. Bbl005229
763. Ccg-38901
764. Codrix Component Acetaminophen
765. Femcet Component Acetaminophen
766. Lortab Component Acetaminophen
767. Norcet Component Acetaminophen
768. Nsc109028
769. Nsc755853
770. Oxycet Component Acetaminophen
771. Paracetamolum [who-ip Latin]
772. Stl140694
773. Tencon Component Acetaminophen
774. Trezix Component Acetaminophen
775. Tylenol Children's Suspension Liquid
776. Zinc13550868
777. Zydone Component Acetaminophen
778. Anexsia Component Acetaminophen
779. Anoquan Component Acetaminophen
780. Butapap Component Acetaminophen
781. Roxicet Component Acetaminophen
782. Roxilox Component Acetaminophen
783. Sedapap Component Acetaminophen
784. Talacen Component Acetaminophen
785. Tycolet Component Acetaminophen
786. Vicodin Component Acetaminophen
787. Wygesic Component Acetaminophen
788. Akos000121004
789. Darvocet Component Acetaminophen
790. Excedrin Component Acetaminophen
791. Feverall Sprinkle Caps Junior Strength
792. Fioricet Component Acetaminophen
793. Hy-phen Component Acetaminophen
794. Ornex Severe Cold Formula (salt/mix)
795. Percocet Component Acetaminophen
796. Tox21_110397_1
797. Triaprin Component Acetaminophen
798. Ultracet Component Acetaminophen
799. Xartemis Component Acetaminophen
800. Acetaminophen Component Of Allay
801. Acetaminophen Component Of Bucet
802. Acetaminophen Component Of Esgic
803. Acetaminophen Component Of Norco
804. Acetaminophen Component Of Triad
805. Acetaminophen Component Of Tylox
806. Bayer Select Allergy-sinus (salt/mix)
807. Bcp9000225
808. Co-gesic Component Acetaminophen
809. Db00316
810. Dhc Plus Component Acetaminophen
811. Nsc-755853
812. Paracetamol 100 Microg/ml In Methanol
813. Acetaminophen Component Of Bancap
814. Acetaminophen Component Of Codrix
815. Acetaminophen Component Of Femcet
816. Acetaminophen Component Of Lortab
817. Acetaminophen Component Of Norcet
818. Acetaminophen Component Of Oxycet
819. Acetaminophen Component Of Tencon
820. Acetaminophen Component Of Zydone
821. Bancap Hc Component Acetaminophen
822. Idi1_000660
823. Lorcet-hd Component Acetaminophen
824. Phrenilin Component Acetaminophen
825. Sine-aid, Maximum Strength (salt/mix)
826. Sudafed Severe Cold Formula (salt/mix)
827. Acetaminophen Component Of Anexsia
828. Acetaminophen Component Of Anoquan
829. Acetaminophen Component Of Butapap
830. Acetaminophen Component Of Roxicet
831. Acetaminophen Component Of Roxilox
832. Acetaminophen Component Of Sedapap
833. Acetaminophen Component Of Talacen
834. Acetaminophen Component Of Tycolet
835. Acetaminophen Component Of Vicodin
836. Acetaminophen Component Of Wygesic
837. Ncgc00016361-01
838. Ncgc00016361-02
839. Ncgc00016361-03
840. Ncgc00016361-04
841. Ncgc00016361-05
842. Ncgc00016361-06
843. Ncgc00016361-08
844. Ncgc00016361-09
845. Ncgc00016361-10
846. Ncgc00016361-12
847. Ncgc00016361-13
848. Ncgc00016361-20
849. Ncgc00025267-01
850. Ncgc00025267-02
851. Ncgc00025267-03
852. Ncgc00025267-04
853. Ncgc00025267-05
854. Ncgc00253912-01
855. Ncgc00259479-01
856. Tylenol Junior Strength Chewable Tablets
857. Ac-23969
858. Acetaminophen Component Of Darvocet
859. Acetaminophen Component Of Excedrin
860. Acetaminophen Component Of Fioricet
861. Acetaminophen Component Of Hy-phen
862. Acetaminophen Component Of Percocet
863. Acetaminophen Component Of Triaprin
864. Acetaminophen Component Of Ultracet
865. Acetaminophen Component Of Xartemis
866. Hy-66005
867. Midol Pm Night Time Formula (salt/mix)
868. Sy001162
869. Triaminic Sore Throat Formula (salt/mix)
870. Acetaminophen Component Of Co-gesic
871. Acetaminophen Component Of Dhc Plus
872. Acetaminophen Component Of Phrenilin
873. Bcp0726000305
874. Duradyne Dhc Component Acetaminophen
875. Sbi-0051269.p003
876. Acetaminophen Component Of Bancap Hc
877. Acetaminophen Component Of Lorcet-hd
878. Bayer Select Sinus Pain Relief (salt/mix)
879. Drixoral Plus Component Acetaminophen
880. Ab00051905
881. Aspirin Free Anacin Maximum Strength Caplets
882. Aspirin Free Anacin Maximum Strength Tablets
883. Dolene Ap-65 Component Acetaminophen
884. Ft-0658035
885. Ft-0661041
886. Ft-0661042
887. H0190
888. Medigesic Plus Component Acetaminophen
889. Sine-off Sinus Medicine Caplets (salt/mix)
890. Synalgos-dc-a Component Acetaminophen
891. Acetaminophen Component Of Duradyne Dhc
892. N-(4-hydroxyphenyl)-acetamide-[13c2,15n]
893. Acetaminophen Component Of Dolene Ap-65
894. Acetaminophen Component Of Drixoral Plus
895. Acetaminophen Component Of Synalgos-dc-a
896. C06804
897. D00217
898. Q57055
899. Ab00051905-09
900. Ab00051905_10
901. Acetaminophen Component Of Medigesic Plus
902. Aspirin Free Anacin Maximum Strength Gel Caplets
903. Bayer Select Menstrual Multi-symptom (salt/mix)
904. Contac Cough & Sore Throat Formula (salt/mix)
905. L024125
906. St. Joseph Cold Tablets For Children (salt/mix)
907. Tylenol Extra Strength Adult Liquid Pain Reliever
908. J-001064
909. J-514275
910. Paracetamol (acetaminophen) 1.0 Mg/ml In Methanol
911. Sr-01000597517-1
912. Sr-01000597517-2
913. Sr-01000597517-4
914. Acetaminophenol 4-acetamino Phenol Paracetamol Somedon
915. Brd-k41524689-001-08-6
916. F3096-1731
917. F48b493f-b1fd-410c-aa0a-f40ec71a0689
918. Bayer Select Maximum Strength Headache Pain Relief Formula
919. Paracetamol, British Pharmacopoeia (bp) Reference Standard
920. Paracetamol, European Pharmacopoeia (ep) Reference Standard
921. Acetaminophen Solution, Drug Standard, 1.0 Mg/ml In Methanol
922. Acetaminophen, United States Pharmacopeia (usp) Reference Standard
923. Acetaminophen, Meets Usp Testing Specifications, 98.0-102.0%, Powder
924. Acetaminophen Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
925. Acetaminophen, Pharmaceutical Secondary Standard; Certified Reference Material
926. Paracetamol For Equipment Qualification, Europepharmacopoeia (ep) Reference Standard
| Molecular Weight | 151.16 g/mol |
|---|---|
| Molecular Formula | C8H9NO2 |
| XLogP3 | 0.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 151.063328530 g/mol |
| Monoisotopic Mass | 151.063328530 g/mol |
| Topological Polar Surface Area | 49.3 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 139 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 20 | |
|---|---|
| Drug Name | Acephen |
| PubMed Health | Acetaminophen |
| Drug Classes | Acetaminophen Combination, Analgesic, Antipyretic |
| Active Ingredient | Acetaminophen |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 650mg; 120mg; 325mg |
| Market Status | Over the Counter |
| Company | G And W Labs |
| 2 of 20 | |
|---|---|
| Drug Name | Acetaminophen |
| PubMed Health | Hydrocodone/Acetaminophen (By mouth) |
| Drug Classes | Analgesic, Opioid/Acetaminophen Combination |
| Drug Label | Acetaminophen and codeine phosphate is supplied in tablet form for oral administration.Acetaminophen, 4'-hydroxyacetanilide, a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. It has the |
| Active Ingredient | Acetaminophen |
| Dosage Form | Tablet, extended release; Suppository |
| Route | Rectal; Oral |
| Strength | 650mg; 120mg; 325mg |
| Market Status | Over the Counter |
| Company | Ohm Labs; Perrigo; Perrigo New York; Ranbaxy Labs; Taro Pharms North |
| 3 of 20 | |
|---|---|
| Drug Name | Butapap |
| PubMed Health | Acetaminophen |
| Drug Classes | Acetaminophen Combination, Analgesic, Antipyretic |
| Active Ingredient | butalbital; Acetaminophen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg; 325mg |
| Market Status | Prescription |
| Company | Mikart |
| 4 of 20 | |
|---|---|
| Drug Name | Infants' feverall |
| PubMed Health | Acetaminophen and Codeine |
| Drug Classes | Analgesic, Codeine, Opioid/Acetaminophen Combination |
| Active Ingredient | Acetaminophen |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 80mg |
| Market Status | Over the Counter |
| Company | Taro Pharms North |
| 5 of 20 | |
|---|---|
| Drug Name | Neopap |
| Active Ingredient | Acetaminophen |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 120mg |
| Market Status | Over the Counter |
| Company | Polymedica |
| 6 of 20 | |
|---|---|
| Drug Name | Norco |
| Drug Label | Acetaminophen is a non-salicylate antipyretic and non-opioid analgesic agent. Its chemical name is N-acetyl-p-aminophenol. Acetaminophen has a molecular weight of 151.16. Its structural formula is: OFIRMEV injection is a sterile, clear, colorless, no... |
| Active Ingredient | hydrocodone bitartrate; Acetaminophen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 7.5mg; 5mg; 10mg; 325mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl; Watson Labs |
| 7 of 20 | |
|---|---|
| Drug Name | Ofirmev |
| Active Ingredient | Acetaminophen |
| Dosage Form | Solution |
| Route | Iv (infusion) |
| Strength | 1000mg/100ml (10mg/ml) |
| Market Status | Prescription |
| Company | Cadence Pharms |
| 8 of 20 | |
|---|---|
| Drug Name | Oxycet |
| Active Ingredient | oxycodone hydrochloride; Acetaminophen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 325mg |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 9 of 20 | |
|---|---|
| Drug Name | Percocet |
| Active Ingredient | oxycodone hydrochloride; Acetaminophen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 7.5mg; 5mg; 10mg; 325mg |
| Market Status | Prescription |
| Company | Vintage Pharms |
| 10 of 20 | |
|---|---|
| Drug Name | Tylenol |
| Active Ingredient | Acetaminophen |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 650mg |
| Market Status | Over the Counter |
| Company | Mcneil Cons |
| 11 of 20 | |
|---|---|
| Drug Name | Acephen |
| PubMed Health | Acetaminophen |
| Drug Classes | Acetaminophen Combination, Analgesic, Antipyretic |
| Active Ingredient | Acetaminophen |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 650mg; 120mg; 325mg |
| Market Status | Over the Counter |
| Company | G And W Labs |
| 12 of 20 | |
|---|---|
| Drug Name | Acetaminophen |
| PubMed Health | Hydrocodone/Acetaminophen (By mouth) |
| Drug Classes | Analgesic, Opioid/Acetaminophen Combination |
| Drug Label | Acetaminophen and codeine phosphate is supplied in tablet form for oral administration.Acetaminophen, 4'-hydroxyacetanilide, a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. It has the |
| Active Ingredient | Acetaminophen |
| Dosage Form | Tablet, extended release; Suppository |
| Route | Rectal; Oral |
| Strength | 650mg; 120mg; 325mg |
| Market Status | Over the Counter |
| Company | Ohm Labs; Perrigo; Perrigo New York; Ranbaxy Labs; Taro Pharms North |
| 13 of 20 | |
|---|---|
| Drug Name | Butapap |
| PubMed Health | Acetaminophen |
| Drug Classes | Acetaminophen Combination, Analgesic, Antipyretic |
| Active Ingredient | butalbital; Acetaminophen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg; 325mg |
| Market Status | Prescription |
| Company | Mikart |
| 14 of 20 | |
|---|---|
| Drug Name | Infants' feverall |
| PubMed Health | Acetaminophen and Codeine |
| Drug Classes | Analgesic, Codeine, Opioid/Acetaminophen Combination |
| Active Ingredient | Acetaminophen |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 80mg |
| Market Status | Over the Counter |
| Company | Taro Pharms North |
| 15 of 20 | |
|---|---|
| Drug Name | Neopap |
| Active Ingredient | Acetaminophen |
| Dosage Form | Suppository |
| Route | Rectal |
| Strength | 120mg |
| Market Status | Over the Counter |
| Company | Polymedica |
| 16 of 20 | |
|---|---|
| Drug Name | Norco |
| Drug Label | Acetaminophen is a non-salicylate antipyretic and non-opioid analgesic agent. Its chemical name is N-acetyl-p-aminophenol. Acetaminophen has a molecular weight of 151.16. Its structural formula is: OFIRMEV injection is a sterile, clear, colorless, no... |
| Active Ingredient | hydrocodone bitartrate; Acetaminophen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 7.5mg; 5mg; 10mg; 325mg |
| Market Status | Prescription |
| Company | Actavis Labs Fl; Watson Labs |
| 17 of 20 | |
|---|---|
| Drug Name | Ofirmev |
| Active Ingredient | Acetaminophen |
| Dosage Form | Solution |
| Route | Iv (infusion) |
| Strength | 1000mg/100ml (10mg/ml) |
| Market Status | Prescription |
| Company | Cadence Pharms |
| 18 of 20 | |
|---|---|
| Drug Name | Oxycet |
| Active Ingredient | oxycodone hydrochloride; Acetaminophen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 325mg |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 19 of 20 | |
|---|---|
| Drug Name | Percocet |
| Active Ingredient | oxycodone hydrochloride; Acetaminophen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 7.5mg; 5mg; 10mg; 325mg |
| Market Status | Prescription |
| Company | Vintage Pharms |
| 20 of 20 | |
|---|---|
| Drug Name | Tylenol |
| Active Ingredient | Acetaminophen |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 650mg |
| Market Status | Over the Counter |
| Company | Mcneil Cons |
Analgesics, Non-Narcotic; Antipyretics
National Library of Medicine's Medical Subject Headings. Acetaminophen. Online file (MeSH, 2014). Available from, as of January 30, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Ofirmev (acetaminophen) injection is indicated for the management of mild to moderate pain the management of moderate to severe pain with adjunctive opioid analgesics the reduction of fever. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for OFIRMEV (acetaminophen) injection, solution (October 2013). Available from, as of March 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=c5177abd-9465-40d8-861d-3904496d82b7
Acetaminophen is used to provide temporary analgesia in the treatment of mild to moderate pain. Acetaminophen also is used in fixed combination with other agents (e.g., chlorpheniramine, dextromethorphan, diphenhydramine, doxylamine, guaifenesin, phenylephrine, pseudoephedrine) for short-term relief of minor aches and pain, headache, and/or other symptoms (e.g., rhinorrhea, sneezing, lacrimation, itching eyes, oronasopharyngeal itching, nasal congestion, cough) associated with seasonal allergic rhinitis (e.g., hay fever), other upper respiratory allergies, or the common cold.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2206
Acetaminophen has been used in the treatment of pain in various combinations with aspirin, caffeine, opiates, and/or other agents. Acetaminophen ... in combination with oral doses of an opiate (e.g., codeine, oxycodone) produces greater analgesic effect than that produced by either acetaminophen or higher doses of the opiate alone.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2206
For more Therapeutic Uses (Complete) data for ACETAMINOPHEN (11 total), please visit the HSDB record page.
The U.S. Food and Drug Administration (FDA) is informing the public that acetaminophen has been associated with a risk of rare but serious skin reactions. These skin reactions, known as Stevens-Johnson Syndrome (SJS), toxic epidermal necrolysis (TEN), and acute generalized exanthematous pustulosis (AGEP), can be fatal. Acetaminophen is a common active ingredient to treat pain and reduce fever; it is included in many prescription and over-the-counter (OTC) products. Reddening of the skin, rash, blisters, and detachment of the upper surface of the skin can occur with the use of drug products that contain acetaminophen. These reactions can occur with first-time use of acetaminophen or at any time while it is being taken. ... Anyone who develops a skin rash or reaction while using acetaminophen or any other pain reliever/fever reducer should stop the drug and seek medical attention right away. Anyone who has experienced a serious skin reaction with acetaminophen should not take the drug again and should contact their health care professional to discuss alternative pain relievers/fever reducers. Health care professionals should be aware of this rare risk and consider acetaminophen, along with other drugs already known to have such an association, when assessing patients with potentially drug-induced skin reactions.
US FDA; FDA Drug Safety Communication: FDA Warns of Rare but Serious Skin Reactions with the Pain Reliever/Fever Reducer Acetaminophen (8/1/2013). Available from, as of March 6, 2014: https://www.fda.gov/drugs/drugsafety/ucm363041.htm
FDA is recommending health care professionals discontinue prescribing and dispensing prescription combination drug products that contain more than 325 milligrams (mg) of acetaminophen1 per tablet, capsule, or other dosage unit. There are no available data to show that taking more than 325 mg of acetaminophen per dosage unit provides additional benefit that outweighs the added risks for liver injury. Further, limiting the amount of acetaminophen per dosage unit will reduce the risk of severe liver injury from inadvertent acetaminophen overdose, which can lead to liver failure, liver transplant, and death.
US FDA; FDA Drug Safety and Availability: FDA recommends health care professionals discontinue prescribing and dispensing prescription combination drug products with more than 325 mg of acetaminophen to protect consumers (1/14/2014). Available from, as of March 9, 2014: https://www.fda.gov/Drugs/DrugSafety/ucm381644.htm
/BOXED WARNING/ WARNING: RISK OF MEDICATION ERRORS AND HEPATOTOXICITY. Take care when prescribing, preparing, and administering Ofirmev Injection to avoid dosing errors which could result in accidental overdose and death. In particular, be careful to ensure that: the dose in milligrams (mg) and milliliters (mL) is not confused; the dosing is based on weight for patients under 50 kg; infusion pumps are properly programmed; and the total daily dose of acetaminophen from all sources does not exceed maximum daily limits. Ofirmev contains acetaminophen. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed the maximum daily limits, and often involve more than one acetaminophen-containing product.
US Natl Inst Health; DailyMed. Current Medication Information for OFIRMEV (acetaminophen) injection, solution (October 2013). Available from, as of March 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=c5177abd-9465-40d8-861d-3904496d82b7
Use caution when administering acetaminophen in patients with the following conditions: hepatic impairment or active hepatic disease, alcoholism, chronic malnutrition, severe hypovolemia (e.g., due to dehydration or blood loss), or severe renal impairment (creatinine clearance = 30 mL/min).
US Natl Inst Health; DailyMed. Current Medication Information for OFIRMEV (acetaminophen) injection, solution (October 2013). Available from, as of March 6, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=c5177abd-9465-40d8-861d-3904496d82b7
For more Drug Warnings (Complete) data for ACETAMINOPHEN (23 total), please visit the HSDB record page.
In adults, hepatic toxicity rarely has occurred with acute overdoses of less than 10 g, although hepatotoxicity has been reported in fasting patients ingesting 4-10 g of acetaminophen. Fatalities are rare with less than 15 g.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2210
In general, acetaminophen is used for the treatment of mild to moderate pain and reduction of fever. It is available over the counter in various forms, the most common being oral forms. Acetaminophen _injection_ is indicated for the management of mild to moderate pain, the management of moderate to severe pain with adjunctive opioid analgesics, and the reduction of fever. Because of its low risk of causing allergic reactions, this drug can be administered in patients who are intolerant to salicylates and those with allergic tendencies, including bronchial asthmatics. Specific dosing guidelines should be followed when administering acetaminophen to children.
FDA Label
Moderate pain and fever
Animal and clinical studies have determined that acetaminophen has both antipyretic and analgesic effects. This drug has been shown to lack anti-inflammatory effects. As opposed to the _salicylate_ drug class, acetaminophen does not disrupt tubular secretion of uric acid and does not affect acid-base balance if taken at the recommended doses. Acetaminophen does not disrupt hemostasis and does not have inhibitory activities against platelet aggregation. Allergic reactions are rare occurrences following acetaminophen use.
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
Antipyretics
Drugs that are used to reduce body temperature in fever. (See all compounds classified as Antipyretics.)
N02BE01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N02 - Analgesics
N02B - Other analgesics and antipyretics
N02BE - Anilides
N02BE01 - Paracetamol
Absorption
Acetaminophen has 88% oral bioavailability and reaches its highest plasma concentration 90 minutes after ingestion. Peak blood levels of free acetaminophen are not reached until 3 hours after rectal administration of the suppository form of acetaminophen and the peak blood concentration is approximately 50% of the observed concentration after the ingestion of an equivalent oral dose (10-20 mcg/mL). The percentage of a systemically absorbed rectal dose of acetaminophen is inconsistent, demonstrated by major differences in the bioavailability of acetaminophen after a dose administered rectally. Higher rectal doses or an increased frequency of administration may be used to attain blood concentrations of acetaminophen similar to those attained after oral acetaminophen administration.
Route of Elimination
Acetaminophen metabolites are mainly excreted in the urine. Less than 5% is excreted in the urine as free (unconjugated) acetaminophen and at least 90% of the administered dose is excreted within 24 hours.
Volume of Distribution
Volume of distribution is about 0.9L/kg. 10 to 20% of the drug is bound to red blood cells. Acetaminophen appears to be widely distributed throughout most body tissues except in fat.
Clearance
Adults: 0.27 L/h/kg following a 15 mg/kg intravenous (IV) dose. Children: 0.34 L/h/kg following a 15 mg/kg intravenous (IV dose).
Acetaminophen is rapidly and almost completely absorbed from the GI tract following oral administration. In healthy men, steady-state oral bioavailability of 1.3-g doses of extended-release tablets of acetaminophen administered every 8 hours for a total of 7 doses was equal to 1-g doses of conventional tablets of acetaminophen given every 6 hours for a total of 7 doses. Food may delay slightly absorption of extended-release tablets of acetaminophen. Following oral administration of immediate- or extended-release acetaminophen preparations, peak plasma concentrations are attained within 10-60 or 60-120 minutes, respectively. Following oral administration of a single 500-mg conventional tablet or a single 650-mg extended-release tablet, average plasma acetaminophen concentrations of 2.1 or 1.8 ug/mL, respectively, occur at 6 or 8 hours, respectively. In addition, dissolution of the extended-release tablets may depend slightly on the gastric or intestinal pH. Dissolution appears to be slightly faster in the alkaline pH of the intestines compared with the acidic pH of the stomach; however, this is of no clinical importance. Following administration of conventional preparations of acetaminophen, only small amounts of the drug are detectable in plasma after 8 hours. The extended-release tablets of acetaminophen release the drug for up to 8 hours, but in vitro data indicate that at least 95% of the dose is released within 5 hours.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2212
Following rectal administration of acetaminophen, there is considerable variation in peak plasma concentrations attained, and time to reach peak plasma concentrations is substantially longer than after oral administration.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2212
In 12 nursing mothers (nursing 2-22 months) given a single oral dose of 650 mg, peak levels of acetaminophen occurred at 1-2 hours in the range of 10-15 ug/mL. Assuming 90 mL of milk were ingested at 3-, 6-, and 9-hour intervals after ingestion, the amount of drug available to the infant was estimated to range from 0.04% to 0.23% of the maternal dose.
Briggs, G.G., Freeman, R.K., Yaffee, S.J.; Drugs in Pregancy and Lactation Nineth Edition. Wolters Kluwer/Lippincott Williams & Wilkins, Philadelphia, PA. 2011, p. 11
Acetaminophen is rapidly and uniformly distributed into most body tissues. About 25% of acetaminophen in blood is bound to plasma proteins.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2212
For more Absorption, Distribution and Excretion (Complete) data for ACETAMINOPHEN (11 total), please visit the HSDB record page.
Acetaminophen is the major metabolite of _phenacetin_ and _acetanilid_. Acetaminophen is mainly metabolized in the liver by first-order kinetics and its metabolism of comprised of 3 pathways: conjugation with glucuronide, conjugation with sulfate, and oxidation through the cytochrome P450 enzyme pathway, mainly CYP2E1, to produce a reactive metabolite (N-acetyl-p-benzoquinone imine or NAPQI). At normal therapeutic doses, NAPQI undergoes fast conjugation with glutathione and is subsequently metabolized to produce both cysteine and mercapturic acid conjugates. High doses of acetaminophen (overdoses) can lead to hepatic necrosis due to the depletion of glutathione and of binding of high levels of reactive metabolite (NAPQI) to important parts of liver cells. The abovementioned damage to the liver can be prevented by the early administration of sulfhydryl compounds, for example, methionine and N-acetylcysteine.
About 80-85% of the acetaminophen in the body undergoes conjugation principally with glucuronic acid and to a lesser extent with sulfuric acid. Acetaminophen also is metabolized by microsomal enzyme systems in the liver.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2212
In vitro and animal data indicate that small quantities of acetaminophen are metabolized by a cytochrome P-450 microsomal enzyme to a reactive intermediate metabolite (N-acetyl-p-benzoquinoneimine, N-acetylimidoquinone, NAPQI) which is further metabolized via conjugation with glutathione and ultimately excreted in urine as a mercapturic acid. It has been suggested that this intermediate metabolite is responsible for acetaminophen-induced liver necrosis and that high doses of acetaminophen may deplete glutathione so that inactivation of this toxic metabolite is decreased. At high doses, the capacity of metabolic pathways for conjugation with glucuronic acid and sulfuric acid may be exceeded, resulting in increased metabolism of acetaminophen by alternative pathways. In addition, it also has been suggested that in fasting individuals conjugation of high doses of acetaminophen with glucuronic acid may be reduced, secondary to decreased hepatic carbohydrate reserves and microsomal oxidation may be increased, resulting in increased risk of hepatotoxicity.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2212
Yields 4-acetamidocatechol in rat; yields s-(5-acetamido-2-hydroxyphenyl)-l-cysteine probably in man. Yields p-acetamidophenyl-beta-d-glucuronide in rabbit; yields p-acetamidophenyl-beta-d-glucuronide in rat, in guinea pig, & in ferret; yields p-acetamidophenyl-beta-d-glucuronide in man & in dog; yields p-acetamidophenyl sulfate in rabbit, guinea pig, & ferret; yields p-acetamidophenyl sulfate in rat & in man; yields p-methoxyacetanilide in guinea pig; yields quinol probably in rat. /From table/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. A-10
Children have less capacity for glucuronidation of the drug than do adults. A small proportion of acetaminophen undgoes n-hydroxylation to form n-acetyl-benzoquinoneimine, a highly reactive intermediate. This metabolite normally reacts with sulfhydryl groups in glutathione. However, after large doses of acetaminophen the metabolite is formed in amounts sufficient to deplete hepatic glutathione; under these circumstances reaction with sulfhydryl groups in hepatic proteins is increased and hepatic necrosis can result.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 704
For more Metabolism/Metabolites (Complete) data for ACETAMINOPHEN (7 total), please visit the HSDB record page.
Acetaminophen has known human metabolites that include (2S,3S,4S,5R)-6-(4-Acetamidophenoxy)-3,4,5-trihydroxyoxane-2-carboxylic acid, 3-Hydroxyacetaminophen, Acetaminophen sulfate, and N-Acetyl-p-benzoquinone.
Acetaminophen is a known human metabolite of O-isopropyl acetaminophen, acetanilide, p-Methoxyacetanilide, and phenacetin.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half-life for adults is 2.5 h after an intravenous dose of 15 mg/kg. After an overdose, the half-life can range from 4 to 8 hours depending on the severity of injury to the liver, as it heavily metabolizes acetaminophen.
The elimination half life is 1-3 hours after a therapeutic dose but may be greater than 12 hours after an overdose.
OLSON, K.R. (Ed). Poisoning and Drug Overdose, Sixth Edition. McGraw-Hill, New York, NY 2012, p. 69
According to its FDA labeling, acetaminophen's exact mechanism of action has not been fully established - despite this, it is often categorized alongside NSAIDs (nonsteroidal anti-inflammatory drugs) due to its ability to inhibit the cyclooxygenase (COX) pathways. It is thought to exert central actions which ultimately lead to the alleviation of pain symptoms. One theory is that acetaminophen increases the pain threshold by inhibiting two isoforms of cyclooxygenase, COX-1 and COX-2, which are involved in prostaglandin (PG) synthesis. Prostaglandins are responsible for eliciting pain sensations. Acetaminophen does not inhibit cyclooxygenase in peripheral tissues and, therefore, has no peripheral anti-inflammatory effects. Though acetylsalicylic acid (aspirin) is an irreversible inhibitor of COX and directly blocks the active site of this enzyme, studies have shown that acetaminophen (paracetamol) blocks COX indirectly. Studies also suggest that acetaminophen selectively blocks a variant type of the COX enzyme that is unique from the known variants COX-1 and COX-2. This enzyme has been referred to as _COX-3_. The antipyretic actions of acetaminophen are likely attributed to direct action on heat-regulating centers in the brain, resulting in peripheral vasodilation, sweating, and loss of body heat. The exact mechanism of action of this drug is not fully understood at this time, but future research may contribute to deeper knowledge.
Acetaminophen produces analgesia and antipyresis by a mechanism similar to that of salicylates. Unlike salicylates, however, acetaminophen does not have uricosuric activity. There is some evidence that acetaminophen has weak anti-inflammatory activity in some nonrheumatoid conditions (e.g., in patients who have had oral surgery). ... Acetaminophen lowers body temperature in patients with fever but rarely lowers normal body temperature. The drug acts on the hypothalamus to produce antipyresis; heat dissipation is increased as a result of vasodilation and increased peripheral blood flow.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2211
The effects of acetaminophen on cyclooxygenase activity have not been fully determined. Acetaminophen is a weak, reversible, isoform-nonspecific cyclooxygenase inhibitor at dosages of 1 g daily. The inhibitory effect of acetaminophen on cyclooxygenase-1 is limited, and the drug does not inhibit platelet function. Therapeutic doses of acetaminophen appear to have little effect on cardiovascular and respiratory systems; however, toxic doses may cause circulatory failure and rapid, shallow breathing.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 2211
Acetaminophen (N-acetyl-p-aminophenol (APAP)) is the most common antipyretic/analgesic medicine worldwide. If APAP is overdosed, its metabolite, N-acetyl-p-benzo-quinoneimine (NAPQI), causes liver damage. However, epidemiological evidence has associated previous use of therapeutic APAP doses with the risk of chronic obstructive pulmonary disease (COPD) and asthma. The transient receptor potential ankyrin-1 (TRPA1) channel is expressed by peptidergic primary sensory neurons. Because NAPQI, like other TRPA1 activators, is an electrophilic molecule, /the researchers/ hypothesized that APAP, via NAPQI, stimulates TRPA1, thus causing airway neurogenic inflammation. NAPQI selectively excites human recombinant and native (neuroblastoma cells) TRPA1. TRPA1 activation by NAPQI releases proinflammatory neuropeptides (substance P and calcitonin gene-related peptide) from sensory nerve terminals in rodent airways, thereby causing neurogenic edema and neutrophilia. Single or repeated administration of therapeutic (15-60 mg/kg) APAP doses to mice produces detectable levels of NAPQI in the lung, and increases neutrophil numbers, myeloperoxidase activity, and cytokine and chemokine levels in the airways or skin. Inflammatory responses evoked by NAPQI and APAP are abated by TRPA1 antagonism or are absent in TRPA1-deficient mice. This novel pathway, distinguished from the tissue-damaging effect of NAPQI, may contribute to the risk of COPD and asthma associated with therapeutic APAP use.
PMID:20720158 Nassini R et al; FASEB J 24 (12): 4904-16 (2010)
Acetaminophen is at present one of the most commonly used analgesics and antipyretics. Recent evidence has suggested that oxidative stress is involved in the mechanism of acetaminophen intoxication. Paraoxonase-1 (PON1) plays an important role as an endogenous free-radical scavenging molecule. The aim of this study was to evaluate the influence of serum PON1 activity and oxidative stress in patients with acetaminophen intoxication. A total of 20 patients with acetaminophen intoxication and 25 healthy controls were enrolled. Serum total antioxidant capacity (TAC), lipid hydroperoxide (LOOH) levels, and paraoxonase and arylesterase activities were measured spectrophotometrically. The serum TAC levels and the paraoxonase and arylesterase activities were significantly lower in patients with acetaminophen intoxication compared with controls (all, p < 0.001), while the serum LOOH levels were significantly higher (p < 0.001). Results suggest that decreased PON1 activity seems to be associated with increased oxidative stress in patients with acetaminophen intoxication. Measuring serum PON1 activity may be useful in assessing the development of toxicity risk in acetaminophen toxicity. It would be useful to recommend vitamins with antioxidant effects such as vitamins C and E along with medical treatments.
PMID:24501102 Karadas S et al; Hum Exp Toxicol 33 (11): 1134-40 (2014)
For more Mechanism of Action (Complete) data for ACETAMINOPHEN (9 total), please visit the HSDB record page.
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17722
Submission : 2004-09-22
Status : Active
Type : II

Registration Number : 228MF10061
Registrant's Address : 2nd Floor, 3rd Block, My Home Hub, Madhapur, Hyderabad, Telangana 500081 India
Initial Date of Registration : 2016-02-24
Latest Date of Registration :

Date of Issue : 2025-08-01
Valid Till : 2028-07-02
Written Confirmation Number : WC-0116
Address of the Firm :

NDC Package Code : 62207-934
Start Marketing Date : 2025-04-14
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Insung Trading Co., Ltd.
Registration Date : 2023-03-29
Registration Number : 20050915-32-C-198-10(B)
Manufacturer Name : Granules India Ltd
Manufacturer Address : H.No.6-5 & 6-11, Temple Road, Bonthapally Village, Gummadidala Mandal, Sangareddy Dist-502313, Telangana State, India.

| Available Reg Filing : ASMF, CA, MX |

GDUFA
DMF Review : Reviewed
Rev. Date : 2025-02-28
Pay. Date : 2025-01-23
DMF Number : 37336
Submission : 2022-07-28
Status : Active
Type : II

Certificate Number : R0-CEP 2022-085 - Rev 00
Issue Date : 2023-05-03
Type : Chemical
Substance Number : 49
Status : Valid

Date of Issue : 2025-02-27
Valid Till : 2028-03-17
Written Confirmation Number : WC-0004
Address of the Firm :

NDC Package Code : 49716-327
Start Marketing Date : 2023-10-15
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Toru Co., Ltd.
Registration Date : 2025-02-03
Registration Number : 20250203-32-C-419-27
Manufacturer Name : IOL Chemicals and Pharmaceuticals Ltd
Manufacturer Address : Village Fatehgarh Channa,Mansa Road, Trident Complex,Barnala-148101, Punjab, INDIA

 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 3142
Submission : 1978-02-01
Status : Inactive
Type : II

 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.

NDC Package Code : 58103-115
Start Marketing Date : 2018-09-05
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (92kg/100kg)
Marketing Category : DRUG FOR FURTHER PROCESSING
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.

Registration Number : 307MF10114
Registrant's Address : No. 16 Guangshi Road, Meijing Village, Luoshe Town, Huishan District, Wuxi
Initial Date of Registration : 2025-09-11
Latest Date of Registration :

 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Certificate Number : R0-CEP 2022-085 - Rev 00
Status : Valid
Issue Date : 2023-05-03
Type : Chemical
Substance Number : 49
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2011-278 - Rev 00
Status : Expired
Issue Date : 2012-03-30
Type : Chemical
Substance Number : 49

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2001-092 - Rev 02
Status : Withdrawn by Holder
Issue Date : 2004-10-13
Type : Chemical
Substance Number : 49

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2005-032 - Rev 04
Status : Valid
Issue Date : 2021-02-15
Type : Chemical
Substance Number : 49

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2000-002 - Rev 00
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2001-10-22
Type : Chemical
Substance Number : 49

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2007-054 - Rev 03
Status : Withdrawn by EDQM GMP non-compliance
Issue Date : 2019-11-06
Type : Chemical
Substance Number : 49

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1996-039 - Rev 04
Status : Valid
Issue Date : 2018-04-25
Type : Chemical
Substance Number : 49

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R2-CEP 1992-010 - Rev 02
Status : Withdrawn by Holder
Issue Date : 2006-08-31
Type : Chemical
Substance Number : 49

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1996-004 - Rev 02
Status : Withdrawn by Holder
Issue Date : 2005-02-14
Type : Chemical
Substance Number : 49

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2006-156 - Rev 00
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2008-04-17
Type : Chemical
Substance Number : 49

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]  Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Registration Number : 228MF10061
Registrant's Address : 2nd Floor, 3rd Block, My Home Hub, Madhapur, Hyderabad, Telangana 500081 India
Initial Date of Registration : 2016-02-24
Latest Date of Registration : 2016-02-24
 Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
Seqens is an integrated global leader in pharmaceutical solutions & specialty ingredients, & custom-made solutions to our customers.
Registration Number : 307MF10114
Registrant's Address : No. 16 Guangshi Road, Meijing Village, Luoshe Town, Huishan District, Wuxi
Initial Date of Registration : 2025-09-11
Latest Date of Registration : 2025-09-11
Registration Number : 303MF10081
Registrant's Address : No. 35, Weixu North Road, Anqiu, Shandong, China
Initial Date of Registration : 2021-05-17
Latest Date of Registration : 2021-05-17

Registration Number : 229MF10093
Registrant's Address : Acibadem, Koftunku Sok, No. 1, 34718, Kadikoy, Istanbul, Turkey
Initial Date of Registration : 2017-05-16
Latest Date of Registration : 2017-05-16

Registration Number : 303MF10174
Registrant's Address : Plot No. 14, GIDC Industrial Estate, Nandesari 391340, District Vadodara, Gujarat, In...
Initial Date of Registration : 2021-12-09
Latest Date of Registration : 2021-12-09

Registration Number : 229MF10149
Registrant's Address : West of Guoxin Road, Xijingming Village, Donganzhuang Township, Shenzhou County, Heng...
Initial Date of Registration : 2017-08-02
Latest Date of Registration : 2017-08-02

Registration Number : 229MF10224
Registrant's Address : West of Guoxin Road, Xijingming Village, Donganzhuang Township, Shenzhou County, Heng...
Initial Date of Registration : 2017-12-25
Latest Date of Registration : 2017-12-25

Registration Number : 228MF10014
Registrant's Address : Southeast Xijingming Village, Donganzhuang Township, Shenzhou County Hengshui City, H...
Initial Date of Registration : 2016-01-08
Latest Date of Registration : 2016-01-08

Japanese Pharmacopoeia Acetaminophen
Registration Number : 230MF10132
Registrant's Address : 1-4 Funatsucho, Wakayama City, Wakayama Prefecture
Initial Date of Registration : 2018-09-06
Latest Date of Registration : 2018-09-06

Registration Number : 229MF10214
Registrant's Address : 10-2 Yoshida Shimojima, Higashiosaka City, Osaka Prefecture
Initial Date of Registration : 2017-12-06
Latest Date of Registration : 2017-12-06

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; BUTALBITAL; CAFFEINE
Brand Name : BUTALBITAL, ACETAMINOPHEN AND CAFFEINE
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;50MG;40MG
Packaging :
Approval Date : 2008-12-01
Application Number : 40864
Regulatory Info : RX
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : OTC
Registration Country : USA
Brand Name : ACETAMINOPHEN
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 650MG
Packaging :
Approval Date : 2019-04-16
Application Number : 211544
Regulatory Info : OTC
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
Brand Name : OXYCODONE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;2.5MG
Packaging :
Approval Date : 2019-10-31
Application Number : 211708
Regulatory Info : RX
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
Brand Name : OXYCODONE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;5MG
Packaging :
Approval Date : 2019-10-31
Application Number : 211708
Regulatory Info : RX
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
Brand Name : OXYCODONE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;7.5MG
Packaging :
Approval Date : 2019-10-31
Application Number : 211708
Regulatory Info : RX
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
Brand Name : OXYCODONE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;10MG
Packaging :
Approval Date : 2019-10-31
Application Number : 211708
Regulatory Info : RX
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Brand Name : HYDROCODONE BITARTRATE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;5MG
Packaging :
Approval Date : 2020-01-03
Application Number : 211729
Regulatory Info : RX
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Brand Name : HYDROCODONE BITARTRATE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;7.5MG
Packaging :
Approval Date : 2020-01-03
Application Number : 211729
Regulatory Info : RX
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Brand Name : HYDROCODONE BITARTRATE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;10MG
Packaging :
Approval Date : 2020-01-03
Application Number : 211729
Regulatory Info : RX
Registration Country : USA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Regulatory Info : RX
Registration Country : USA
Brand Name : BUTALBITAL AND ACETAMINOPHEN
Dosage Form : CAPSULE;ORAL
Dosage Strength : 300MG;50MG
Packaging :
Approval Date : 2019-11-22
Application Number : 213115
Regulatory Info : RX
Registration Country : USA
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
ACETAMINOPHEN; BUTALBITAL; CAFFEINE
Brand Name : BUTALBITAL, ACETAMINOPHEN AND CAFFEINE
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;50MG;40MG
Approval Date : 2008-12-01
Application Number : 40864
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code :
Brand Name : ACETAMINOPHEN
Dosage Form : TABLET, EXTENDED RELEASE;ORAL
Dosage Strength : 650MG
Approval Date : 2019-04-16
Application Number : 211544
RX/OTC/DISCN : OTC
RLD : No
TE Code :
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
Brand Name : OXYCODONE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;5MG
Approval Date : 2019-10-31
Application Number : 211708
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
Brand Name : OXYCODONE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;7.5MG
Approval Date : 2019-10-31
Application Number : 211708
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
Brand Name : OXYCODONE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;10MG
Approval Date : 2019-10-31
Application Number : 211708
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Brand Name : HYDROCODONE BITARTRATE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;5MG
Approval Date : 2020-01-03
Application Number : 211729
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Brand Name : HYDROCODONE BITARTRATE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;7.5MG
Approval Date : 2020-01-03
Application Number : 211729
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
ACETAMINOPHEN; HYDROCODONE BITARTRATE
Brand Name : HYDROCODONE BITARTRATE AND ACETAMINOPHEN
Dosage Form : TABLET;ORAL
Dosage Strength : 325MG;10MG
Approval Date : 2020-01-03
Application Number : 211729
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
Brand Name : BUTALBITAL AND ACETAMINOPHEN
Dosage Form : CAPSULE;ORAL
Dosage Strength : 300MG;50MG
Approval Date : 2019-11-22
Application Number : 213115
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AA
ACETAMINOPHEN; BUTALBITAL; CAFFEINE
Brand Name : BUTALBITAL, ACETAMINOPHEN AND CAFFEINE
Dosage Form : CAPSULE;ORAL
Dosage Strength : 300MG;50MG;40MG
Approval Date : 2020-04-08
Application Number : 213321
RX/OTC/DISCN : RX
RLD : No
TE Code : AA
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
23 Feb 2026
Reply
16 Jan 2026
Reply
17 Nov 2025
Reply
15 Nov 2025
Reply
04 Nov 2025
Reply
01 Nov 2025
Reply
27 Oct 2025
Reply
03 Oct 2025
Reply
23 Sep 2025
Reply
21 Aug 2025
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Patents & EXCLUSIVITIES
Patent Expiration Date : 2027-06-01
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
US Patent Number : 7976870
Drug Substance Claim :
Drug Product Claim :
Application Number : 204031
Patent Use Code : U-1498
Delist Requested :
Patent Use Description : METHOD OF TREATING PAT...
Patent Expiration Date : 2027-06-01

Patent Expiration Date : 2029-03-11
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
US Patent Number : 8394408
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 204031
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2029-03-11

Patent Expiration Date : 2032-05-16
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
US Patent Number : 9468636
Drug Substance Claim :
Drug Product Claim :
Application Number : 204031
Patent Use Code : U-1499
Delist Requested :
Patent Use Description : MANAGEMENT OF ACUTE PA...
Patent Expiration Date : 2032-05-16

Patent Expiration Date : 2032-05-16
ACETAMINOPHEN; OXYCODONE HYDROCHLORIDE
US Patent Number : 8992975
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 204031
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2032-05-16

Patent Expiration Date : 2029-05-13
US Patent Number : 10383834*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 22450
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2029-05-13

Patent Expiration Date : 2032-03-11
US Patent Number : 9399012*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 22450
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2032-03-11

Patent Expiration Date : 2028-11-13
US Patent Number : 10383834
Drug Substance Claim :
Drug Product Claim :
Application Number : 22450
Patent Use Code : U-2621
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2028-11-13

Patent Expiration Date : 2029-05-13
US Patent Number : 9987238*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 22450
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2029-05-13

Patent Expiration Date : 2028-11-13
US Patent Number : 10383834
Drug Substance Claim :
Drug Product Claim :
Application Number : 22450
Patent Use Code : U-2262
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2028-11-13

Patent Expiration Date : 2030-07-01
ACETAMINOPHEN; BENZHYDROCODONE HYDROCHLORIDE
US Patent Number : 8828978
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 208653
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-07-01

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : NP
Exclusivity Expiration Date : 2026-03-01
Application Number : 209471
Product Number : 1
Exclusivity Details :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Exclusivity Code : PC
Exclusivity Expiration Date : 2026-01-07
Application Number : 214331
Product Number : 2
Exclusivity Details :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
56
PharmaCompass offers a list of Paracetamol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Paracetamol manufacturer or Paracetamol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Paracetamol manufacturer or Paracetamol supplier.
PharmaCompass also assists you with knowing the Paracetamol API Price utilized in the formulation of products. Paracetamol API Price is not always fixed or binding as the Paracetamol Price is obtained through a variety of data sources. The Paracetamol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Acetaminophen manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Acetaminophen, including repackagers and relabelers. The FDA regulates Acetaminophen manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Acetaminophen API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Acetaminophen manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Acetaminophen supplier is an individual or a company that provides Acetaminophen active pharmaceutical ingredient (API) or Acetaminophen finished formulations upon request. The Acetaminophen suppliers may include Acetaminophen API manufacturers, exporters, distributors and traders.
click here to find a list of Acetaminophen suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Acetaminophen DMF (Drug Master File) is a document detailing the whole manufacturing process of Acetaminophen active pharmaceutical ingredient (API) in detail. Different forms of Acetaminophen DMFs exist exist since differing nations have different regulations, such as Acetaminophen USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Acetaminophen DMF submitted to regulatory agencies in the US is known as a USDMF. Acetaminophen USDMF includes data on Acetaminophen's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Acetaminophen USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Acetaminophen suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Acetaminophen Drug Master File in Japan (Acetaminophen JDMF) empowers Acetaminophen API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Acetaminophen JDMF during the approval evaluation for pharmaceutical products. At the time of Acetaminophen JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Acetaminophen suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Acetaminophen Drug Master File in Korea (Acetaminophen KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Acetaminophen. The MFDS reviews the Acetaminophen KDMF as part of the drug registration process and uses the information provided in the Acetaminophen KDMF to evaluate the safety and efficacy of the drug.
After submitting a Acetaminophen KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Acetaminophen API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Acetaminophen suppliers with KDMF on PharmaCompass.
A Acetaminophen CEP of the European Pharmacopoeia monograph is often referred to as a Acetaminophen Certificate of Suitability (COS). The purpose of a Acetaminophen CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Acetaminophen EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Acetaminophen to their clients by showing that a Acetaminophen CEP has been issued for it. The manufacturer submits a Acetaminophen CEP (COS) as part of the market authorization procedure, and it takes on the role of a Acetaminophen CEP holder for the record. Additionally, the data presented in the Acetaminophen CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Acetaminophen DMF.
A Acetaminophen CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Acetaminophen CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Acetaminophen suppliers with CEP (COS) on PharmaCompass.
A Acetaminophen written confirmation (Acetaminophen WC) is an official document issued by a regulatory agency to a Acetaminophen manufacturer, verifying that the manufacturing facility of a Acetaminophen active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Acetaminophen APIs or Acetaminophen finished pharmaceutical products to another nation, regulatory agencies frequently require a Acetaminophen WC (written confirmation) as part of the regulatory process.
click here to find a list of Acetaminophen suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Acetaminophen as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Acetaminophen API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Acetaminophen as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Acetaminophen and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Acetaminophen NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Acetaminophen suppliers with NDC on PharmaCompass.
Acetaminophen Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Acetaminophen GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Acetaminophen GMP manufacturer or Acetaminophen GMP API supplier for your needs.
A Acetaminophen CoA (Certificate of Analysis) is a formal document that attests to Acetaminophen's compliance with Acetaminophen specifications and serves as a tool for batch-level quality control.
Acetaminophen CoA mostly includes findings from lab analyses of a specific batch. For each Acetaminophen CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Acetaminophen may be tested according to a variety of international standards, such as European Pharmacopoeia (Acetaminophen EP), Acetaminophen JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Acetaminophen USP).

